
Concept explainers
(a)
Interpretation:
The following alcohol should be classified as primary, secondary and tertiary alcohol:
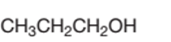
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be
Answer to Problem 14.29P
Propanol is a primary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here, the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group is bonded to a primary C atom which is bonded with one other C atom hence, it should be primary alcohol.
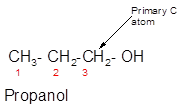
(b)
Interpretation:
The following alcohol should be classified as primary, secondary and tertiary alcohol:
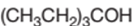
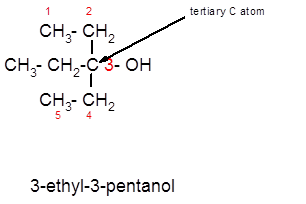
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be organic chemistry. The molecular formula of the organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule.
Answer to Problem 14.29P
3-ethyl-3-pentanol is a tertiary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group is bonded to a tertiary C atom which is bonded with 3 other C atoms hence, it should be tertiary alcohol.
(c)
Interpretation:
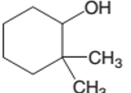
The following alcohol should be classified as primary, secondary and tertiary alcohol:
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be organic chemistry. The molecular formula of the organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule.
Answer to Problem 14.29P
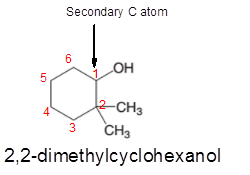
2,2-dimethylcyclohexanol is a secondary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group is bonded to a secondary C atom which is bonded with two other C atoms hence, it should be secondary alcohol.
(d)
Interpretation:
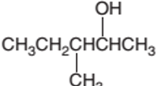
The following alcohol should be classified as primary, secondary and tertiary alcohol:
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be organic chemistry. The molecular formula of the organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule.
Answer to Problem 14.29P
3-methyl-2-pentanol is a secondary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group bonds to a secondary C atom which is bonded with two other C atoms hence it should be secondary alcohol.
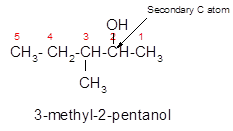
Want to see more full solutions like this?
Chapter 14 Solutions
General, Organic, & Biological Chemistry
- Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$arrow_forwardIndicate characteristics of oxodec acid.arrow_forwardWhat is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forward
- What is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forward
- please help me with my homeworkarrow_forwardhelparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





