
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
3rd Edition
ISBN: 9781259298424
Author: SMITH
Publisher: VST
expand_more
expand_more
format_list_bulleted
Question
Chapter 14.5, Problem 14.10P
Interpretation Introduction
Interpretation:
The reason for readily dehydration reaction shown by givenalcohol A compared to alcohol B should be explained.
Concept Introduction:
When H2O is lost from a material it is known as dehydration. When an alcohol is treated with a strong acid such as H2SO4, a water molecule is lost by breaking two adjacent bonds C-OH and C-H and forming a new double bond, producing an
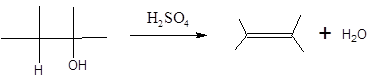
In this reaction, more than one type of alkene may produce. But one of them is the major product. The Zaitsev rule states that the major product in elimination is the alkene that has more alkyl groups bonded to it.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Consider a solution of 0.00304 moles of 4-nitrobenzoic acid (pKa = 3.442) dissolved in 25 mL water and titrated with 0.0991 M NaOH. Calculate the pH at the equivalence point
What is the name of the following compound?
SiMe3
K
Draw the starting structure that would lead to the major
product shown under the provided conditions.
Drawing
1. NaNH2
2. PhCH2Br
4 57°F
Sunny
Q Search
Chapter 14 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
Ch. 14.1 - Prob. 14.1PCh. 14.2 - Prob. 14.2PCh. 14.2 - Classify each hydroxyl group in sorbitol as 1°,...Ch. 14.2 - Prob. 14.4PCh. 14.2 - Prob. 14.5PCh. 14.3 - Prob. 14.6PCh. 14.3 - Give the structure corresponding to each name a....Ch. 14.5 - Draw the products formed when each alcohol is...Ch. 14.5 - Prob. 14.9PCh. 14.5 - Prob. 14.10P
Ch. 14.5 - Prob. 14.11PCh. 14.6 - Prob. 14.12PCh. 14.6 - Prob. 14.13PCh. 14.7 - Prob. 14.14PCh. 14.7 - Prob. 14.15PCh. 14.7 - Prob. 14.16PCh. 14.7 - Prob. 14.17PCh. 14.8 - (a) Translate the hall and stick model of...Ch. 14.8 - Prob. 14.19PCh. 14.9 - Prob. 14.20PCh. 14.9 - Prob. 14.21PCh. 14.9 - Prob. 14.22PCh. 14.9 - Prob. 14.23PCh. 14.9 - Prob. 14.24PCh. 14.9 - Prob. 14.25PCh. 14.10 - Prob. 14.26PCh. 14.10 - Prob. 14.27PCh. 14.10 - Prob. 14.28PCh. 14 - Prob. 14.29PCh. 14 - Prob. 14.30PCh. 14 - Prob. 14.31PCh. 14 - Classify each halide hi A as 1°, 2°, or 3°. A is a...Ch. 14 - Prob. 14.33PCh. 14 - Draw the structure of a molecule that fits each...Ch. 14 - Draw the structure of the six constitutional...Ch. 14 - Draw the structure of the four constitutional...Ch. 14 - Prob. 14.37PCh. 14 - Prob. 14.38PCh. 14 - Prob. 14.39PCh. 14 - Answer each question about alcohol B. Draw a...Ch. 14 - Prob. 14.41PCh. 14 - Give the IUAPC name for each alcohol.Ch. 14 - Prob. 14.43PCh. 14 - Prob. 14.44PCh. 14 - Prob. 14.45PCh. 14 - Prob. 14.46PCh. 14 - Draw the structures and give the IUPAC names for...Ch. 14 - Prob. 14.48PCh. 14 - Prob. 14.49PCh. 14 - Prob. 14.50PCh. 14 - Give the structure corresponding to each IUPAC...Ch. 14 - Give the structure corresponding to each IUPAC...Ch. 14 - Which compound in each pair has the higher boiling...Ch. 14 - Rank the compounds in order of increasing melting...Ch. 14 - Rank the following compounds in order of...Ch. 14 - Rank the following compounds in order of...Ch. 14 - Prob. 14.57PCh. 14 - Prob. 14.58PCh. 14 - Prob. 14.59PCh. 14 - Prob. 14.60PCh. 14 - Prob. 14.61PCh. 14 - Prob. 14.62PCh. 14 - Prob. 14.63PCh. 14 - Prob. 14.64PCh. 14 - Prob. 14.65PCh. 14 - Prob. 14.66PCh. 14 - Prob. 14.67PCh. 14 - Prob. 14.68PCh. 14 - Prob. 14.69PCh. 14 - Prob. 14.70PCh. 14 - Prob. 14.71PCh. 14 - Prob. 14.72PCh. 14 - Prob. 14.73PCh. 14 - Prob. 14.74PCh. 14 - Prob. 14.75PCh. 14 - Prob. 14.76PCh. 14 - Prob. 14.77PCh. 14 - Prob. 14.78PCh. 14 - Prob. 14.79PCh. 14 - Prob. 14.80PCh. 14 - Prob. 14.81PCh. 14 - Prob. 14.82PCh. 14 - Prob. 14.83PCh. 14 - Prob. 14.84PCh. 14 - Prob. 14.85PCh. 14 - Prob. 14.86PCh. 14 - With reference to the halogenated organic...Ch. 14 - Prob. 14.88PCh. 14 - Prob. 14.89PCh. 14 - Prob. 14.90PCh. 14 - Write out the chemical reaction that occurs when a...Ch. 14 - Prob. 14.92PCh. 14 - Prob. 14.93PCh. 14 - Lactic acid [CH3CH(OH)CO2H] gives sour milk its...Ch. 14 - Prob. 14.95PCh. 14 - Prob. 14.96PCh. 14 - Prob. 14.97PCh. 14 - Prob. 14.98PCh. 14 - Prob. 14.99PCh. 14 - Answer the following questions about alcohol B....Ch. 14 - Prob. 14.101CPCh. 14 - Dehydration of alcohol C forms two products of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forwardIndicate the compound formula: dimethyl iodide (propyl) sulfonium.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY