
Concept explainers
(a)
Interpretation:
The following alcohol should be classified as primary, secondary and tertiary alcohol:
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be
Answer to Problem 14.29P
Propanol is a primary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here, the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group is bonded to a primary C atom which is bonded with one other C atom hence, it should be primary alcohol.
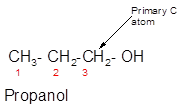
(b)
Interpretation:
The following alcohol should be classified as primary, secondary and tertiary alcohol:
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be organic chemistry. The molecular formula of the organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule.
Answer to Problem 14.29P
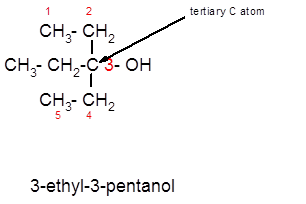
3-ethyl-3-pentanol is a tertiary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group is bonded to a tertiary C atom which is bonded with 3 other C atoms hence, it should be tertiary alcohol.
(c)
Interpretation:
The following alcohol should be classified as primary, secondary and tertiary alcohol:
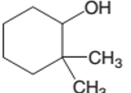
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be organic chemistry. The molecular formula of the organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule.
Answer to Problem 14.29P
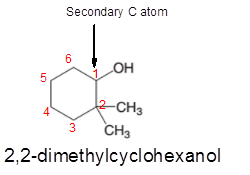
2,2-dimethylcyclohexanol is a secondary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group is bonded to a secondary C atom which is bonded with two other C atoms hence, it should be secondary alcohol.
(d)
Interpretation:
The following alcohol should be classified as primary, secondary and tertiary alcohol:
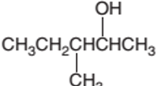
Concept Introduction:
Organic compounds are the compounds that are mainly composed of C and H atoms. The branch of chemistry that deals with the preparation, reactions, and properties of organic compounds are said to be organic chemistry. The molecular formula of the organic compound represents the number of bonded atoms with their atomic symbols. The structural formula represents all the bonded atoms with chemical bonds and the arrangement of atoms in the molecule.
Answer to Problem 14.29P
3-methyl-2-pentanol is a secondary alcohol.
Explanation of Solution
Alcohols are the organic compounds with the general formula R-OH. Here the −OH group (hydroxyl group) is directly bonded to the carbon atom of the parent chain. On the basis of the carbon atom to which the −OH group is bonded, alcohols can be classified as primary, secondary and tertiary alcohols.
- Primary alcohol = The −OH group must be bonded to the primary carbon atom (bonded with one other C atom).
- Secondary alcohol = The −OH group must be bonded to the secondary carbon atom (bonded with two other C atoms).
- Tertiary alcohol = The −OH group must be bonded to a tertiary carbon atom (bonded with three other C atoms).
In the given alcohol, the −OH group bonds to a secondary C atom which is bonded with two other C atoms hence it should be secondary alcohol.
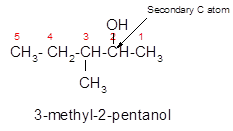
Want to see more full solutions like this?
Chapter 14 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- 23.34 Show how to convert each starting material into isobutylamine in good yield. ཅ ནད ཀྱི (b) Br OEt (c) (d) (e) (f) Harrow_forwardPlease help me Please use https://app.molview.com/ to draw this. I tried, but I couldn't figure out how to do it.arrow_forwardPropose a synthesis of 1-butanamine from the following: (a) a chloroalkane of three carbons (b) a chloroalkane of four carbonsarrow_forward
- Select the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forwardb. H3C CH3 H3O+ ✓ H OHarrow_forward
- 2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forwardIdentify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forward
- Instructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forwardC. NaOMe, Br Brarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





