
Concept explainers
(a)
Interpretation:
The dehydrate product formed from the following alcohol with
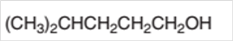
Concept Introduction:
A
In a chemical reaction, the substance which is involved in conversion is said to be a reactant, whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form
Answer to Problem 14.62P
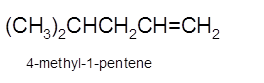
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
Hence, the dehydration of 4-methylpentanol will form 4-methyl-1-pentene molecule.
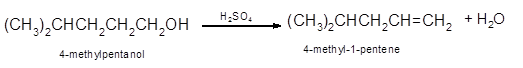
(b)
Interpretation:
The dehydrate product formed from the following alcohol with
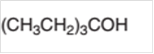
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 14.62P
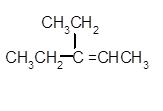
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
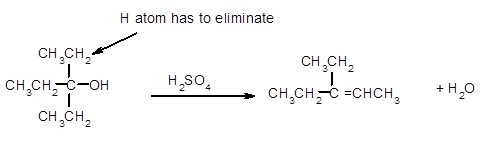
(c)
Interpretation:
The dehydrated product formed from the following alcohol with
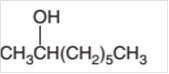
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 14.62P
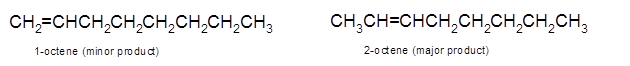
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
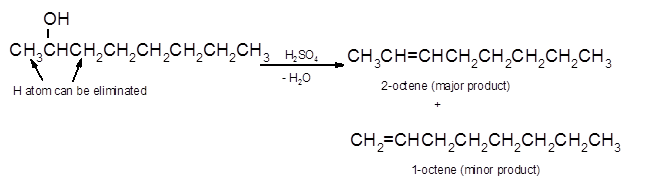
(d)
Interpretation:
The dehydrate product formed from the following alcohol with
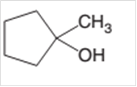
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
A dehydration reaction is an elimination reaction in which a water molecule eliminates from alcohol to form alkene in the presence of
Answer to Problem 14.62P
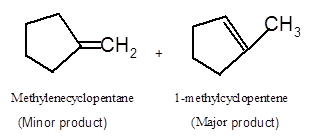
Explanation of Solution
To get the dehydrated product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Eliminate H and OH group from two adjacent C's
- Add a double bond between these C's to form the product alkene.
- If there is a possibility to form two or more alkene, the major product has more C's bonded to the C=C. This is known as the Zaitsev rule.
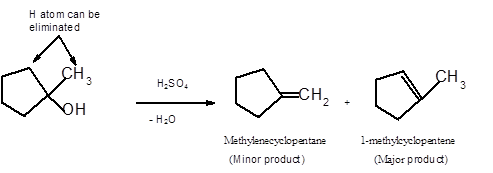
Want to see more full solutions like this?
Chapter 14 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- Beer’s Law is A = εbc, where A is absorbance, ε is the molar absorptivity (which is specific to the compound and wavelength in the measurement), and c is concentration. The absorbance of a 2.31 × 10-5 M solution of a compound is 0.822 at a wavelength of 266 nm in a 1.00-cm cell. Calculate the molar absorptivity at 266 nm.arrow_forwardHow to calculate % of unknown solution using line of best fit y=0.1227x + 0.0292 (y=2.244)arrow_forwardGiven a 1,3-dicarbonyl compound, state the (condensed) formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forward
- Complete the following acid-base reactions and predict the direction of equilibrium for each. Justify your prediction by citing pK values for the acid and conjugate acid in each equilibrium. (a) (b) NHs (c) O₂N NH NH OH H₁PO₁arrow_forward23.34 Show how to convert each starting material into isobutylamine in good yield. ཅ ནད ཀྱི (b) Br OEt (c) (d) (e) (f) Harrow_forwardPlease help me Please use https://app.molview.com/ to draw this. I tried, but I couldn't figure out how to do it.arrow_forward
- Propose a synthesis of 1-butanamine from the following: (a) a chloroalkane of three carbons (b) a chloroalkane of four carbonsarrow_forwardSelect the stronger base from each pair of compounds. (a) H₂CNH₂ or EtzN (b) CI or NH2 NH2 (c) .Q or EtzN (d) or (e) N or (f) H or Harrow_forward4. Provide a clear arrow-pushing mechanism for each of the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. 2. 1. LDA 3. H3O+ HOarrow_forward
- b. H3C CH3 H3O+ ✓ H OHarrow_forward2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forwardIdentify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





