
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
3rd Edition
ISBN: 9781259298424
Author: SMITH
Publisher: VST
expand_more
expand_more
format_list_bulleted
Question
Chapter 14, Problem 14.81P
Interpretation Introduction
(a)
Interpretation:
The disulfide formed on oxidizing the following thiol should be determined:
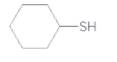
Concept Introduction:
Thiols are organic compounds which contains a sulfhydryl group (SH group) bonded to a tetrahedral carbon.
Reaction of thiols with oxidizing agent will form disulfides and the reaction is known as an oxidation reaction.
Interpretation Introduction
(b)
Interpretation:
The disulfide formed on oxidizing the following thiol should be determined:
Concept Introduction:
Thiols are organic compounds which contains a sulfhydryl group (SH group) bonded to a tetrahedral carbon.
Reaction of thiols with oxidizing agent will form disulfides and the reaction is known as an oxidation reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Rank the labeled protons (Ha-Hd) in order of increasing acidity, starting with the least acidic.
НОН НЬ
OHd
Онс
Can the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left?
?
starting
material
target
If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area.
Be sure you follow the standard ALEKS rules for submitting syntheses.
+ More...
Note for advanced students: you may assume that you are using a large excess of benzene as your starting material.
C
:0
T
Add/Remove step
G
The following equations represent the formation of compound MX. What is the AH for the
electron affinity of X (g)?
X₂ (g) → 2X (g)
M (s) → M (g)
M (g)
M (g) + e-
AH = 60 kJ/mol
AH = 22 kJ/mol
X (g) + e-X (g)
M* (g) +X (g) → MX (s)
AH = 118 kJ/mol
AH = ?
AH = -190 kJ/mol
AH = -100 kJ/mol
a)
-80 kJ
b)
-30 kJ
c)
-20 kJ
d)
20 kJ
e)
156 kJ
Chapter 14 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
Ch. 14.1 - Prob. 14.1PCh. 14.2 - Prob. 14.2PCh. 14.2 - Classify each hydroxyl group in sorbitol as 1°,...Ch. 14.2 - Prob. 14.4PCh. 14.2 - Prob. 14.5PCh. 14.3 - Prob. 14.6PCh. 14.3 - Give the structure corresponding to each name a....Ch. 14.5 - Draw the products formed when each alcohol is...Ch. 14.5 - Prob. 14.9PCh. 14.5 - Prob. 14.10P
Ch. 14.5 - Prob. 14.11PCh. 14.6 - Prob. 14.12PCh. 14.6 - Prob. 14.13PCh. 14.7 - Prob. 14.14PCh. 14.7 - Prob. 14.15PCh. 14.7 - Prob. 14.16PCh. 14.7 - Prob. 14.17PCh. 14.8 - (a) Translate the hall and stick model of...Ch. 14.8 - Prob. 14.19PCh. 14.9 - Prob. 14.20PCh. 14.9 - Prob. 14.21PCh. 14.9 - Prob. 14.22PCh. 14.9 - Prob. 14.23PCh. 14.9 - Prob. 14.24PCh. 14.9 - Prob. 14.25PCh. 14.10 - Prob. 14.26PCh. 14.10 - Prob. 14.27PCh. 14.10 - Prob. 14.28PCh. 14 - Prob. 14.29PCh. 14 - Prob. 14.30PCh. 14 - Prob. 14.31PCh. 14 - Classify each halide hi A as 1°, 2°, or 3°. A is a...Ch. 14 - Prob. 14.33PCh. 14 - Draw the structure of a molecule that fits each...Ch. 14 - Draw the structure of the six constitutional...Ch. 14 - Draw the structure of the four constitutional...Ch. 14 - Prob. 14.37PCh. 14 - Prob. 14.38PCh. 14 - Prob. 14.39PCh. 14 - Answer each question about alcohol B. Draw a...Ch. 14 - Prob. 14.41PCh. 14 - Give the IUAPC name for each alcohol.Ch. 14 - Prob. 14.43PCh. 14 - Prob. 14.44PCh. 14 - Prob. 14.45PCh. 14 - Prob. 14.46PCh. 14 - Draw the structures and give the IUPAC names for...Ch. 14 - Prob. 14.48PCh. 14 - Prob. 14.49PCh. 14 - Prob. 14.50PCh. 14 - Give the structure corresponding to each IUPAC...Ch. 14 - Give the structure corresponding to each IUPAC...Ch. 14 - Which compound in each pair has the higher boiling...Ch. 14 - Rank the compounds in order of increasing melting...Ch. 14 - Rank the following compounds in order of...Ch. 14 - Rank the following compounds in order of...Ch. 14 - Prob. 14.57PCh. 14 - Prob. 14.58PCh. 14 - Prob. 14.59PCh. 14 - Prob. 14.60PCh. 14 - Prob. 14.61PCh. 14 - Prob. 14.62PCh. 14 - Prob. 14.63PCh. 14 - Prob. 14.64PCh. 14 - Prob. 14.65PCh. 14 - Prob. 14.66PCh. 14 - Prob. 14.67PCh. 14 - Prob. 14.68PCh. 14 - Prob. 14.69PCh. 14 - Prob. 14.70PCh. 14 - Prob. 14.71PCh. 14 - Prob. 14.72PCh. 14 - Prob. 14.73PCh. 14 - Prob. 14.74PCh. 14 - Prob. 14.75PCh. 14 - Prob. 14.76PCh. 14 - Prob. 14.77PCh. 14 - Prob. 14.78PCh. 14 - Prob. 14.79PCh. 14 - Prob. 14.80PCh. 14 - Prob. 14.81PCh. 14 - Prob. 14.82PCh. 14 - Prob. 14.83PCh. 14 - Prob. 14.84PCh. 14 - Prob. 14.85PCh. 14 - Prob. 14.86PCh. 14 - With reference to the halogenated organic...Ch. 14 - Prob. 14.88PCh. 14 - Prob. 14.89PCh. 14 - Prob. 14.90PCh. 14 - Write out the chemical reaction that occurs when a...Ch. 14 - Prob. 14.92PCh. 14 - Prob. 14.93PCh. 14 - Lactic acid [CH3CH(OH)CO2H] gives sour milk its...Ch. 14 - Prob. 14.95PCh. 14 - Prob. 14.96PCh. 14 - Prob. 14.97PCh. 14 - Prob. 14.98PCh. 14 - Prob. 14.99PCh. 14 - Answer the following questions about alcohol B....Ch. 14 - Prob. 14.101CPCh. 14 - Dehydration of alcohol C forms two products of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forward
- All of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forward
- Name the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY