
Concept explainers
Classify each example of molecular art as a pure element, a pure compound, or a mixture.
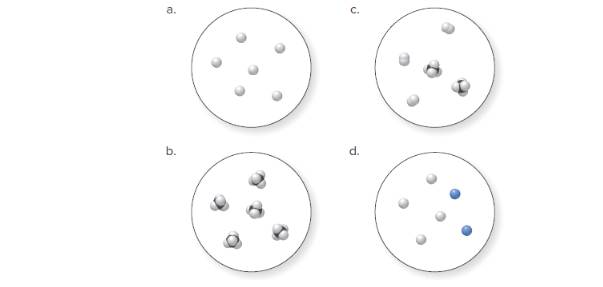
(a)
Interpretation:
Whether the given molecular art is a pure compound, mixture, or a pure element needs to be classified.
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 1.33P
Molecular art 'a' − Pure element.
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now, based on these definitions, molecular art (a) signifies pure elements.
(b)
Interpretation:
Whether the below molecular art is pure compound, mixture, or a pure element needs to be determined.
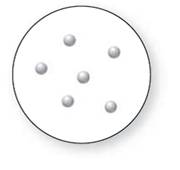
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 1.33P
Molecular art 'b' − Pure compounds
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now based on these definitions, molecular art (b) signifies pure compounds.
(c)
Interpretation:
Whether the below compound is a pure compound, mixture, or a pure element needs to be determined.
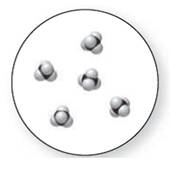
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 1.33P
Molecular art 'c' − Mixture
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now based on these definitions, molecular art (c) signifies mixture.
(c)
Interpretation:
Whether the below compound is a pure compound, mixture, or a pure element needs to be determined.
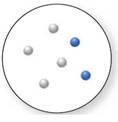
Concept Introduction:
An element is known as the pure substance that cannot be broken down further using chemical methods. The methods are such as electrolysis, cooling, heating, and reactions with other chemical substances.
A compound is known as the pure substance which is made up of more than two different atoms that are bonded chemically to one another. Using chemical methods, a compound can be destroyed. It can be broken down into simpler compounds or into its elements.
A mixture is the combination of more than two dissimilar elemental substances or compounds. The mixture is not a pure substance, but it is the combination of different atoms of elements. Mixtures are of two kinds, Heterogenous and Homogeneous.
Answer to Problem 1.33P
Mixture
Explanation of Solution
As per the definitions of element, compound and mixture:
The pure element is the substance that doesn't separate into simple substances through the chemical procedures.
A pure compound is a substance formed by combinations of two or more elements.
A mixture is made up of the combination of one or more components with several compositions.
Now, based on these definitions, molecular art (d) signifies mixture.
Want to see more full solutions like this?
Chapter 1 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
Additional Science Textbook Solutions
Anatomy & Physiology (6th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Campbell Essential Biology with Physiology (5th Edition)
Organic Chemistry
Campbell Biology (11th Edition)
- Part 1. Draw monomer units of the following products and draw their reaction mechanism 1) Bakelite like polymer Using: Resorcinol + NaOH + Formalin 2) Polyester fiber Using a) pthalic anhydride + anhydrous sodium acetate + ethylene glycol B)pthalic anhydride + anhydrous sodium acetate + glycerol 3) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardUsing the table of Reactants and Products provided provide the correct letter that corresponds with the Carboxylic acid that is formed in the reaction below. 6 M NaOH Acid-workup WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES A) Pool of Reagents for Part B CI B) OH C) E) CI J) racemic F) K) OH N) OH P) G) OH D) HO H) L) M) HO Q) R) CI Aarrow_forwardIn the table below, the exact chemical structures for Methyl salicylate can be represented by the letter WRITE THE CORRECT LETTER ONLY DO NOT WRITE EXTRA WORDS OR PHRASES CI B) A) E) Cl racemic F) J) CI K) N) OH P) Pool of Reagents for Part B OH OH G) L) OH D) HO H) M) HO Q) R) CIarrow_forward
- Draw the stepwise mechanism for the reactionsarrow_forwardPart I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





