
Concept explainers
(a)
Interpretation:
The given diameter 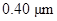 is to be converted to meters and reported in scientific notation.
is to be converted to meters and reported in scientific notation.
Concept introduction:
The scientific notation is the standard method of representation of a long or tedious measurement. The number is expressed as number times ten raised to some power.
For instance mass of a proton is conveniently expressed as 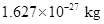 The decimal is shifted to right when the power is positive while it is negative if the decimal has been shifted to left.
The decimal is shifted to right when the power is positive while it is negative if the decimal has been shifted to left.
While a number is written in scientific notations it is scaled down to some digit between 1 to10 ten and the places that previously contain zeroes are changed to ten raised to some power.
For example, 0.00056 is written as 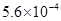
The conversion factor to convert the 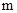 to
to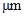 is as follows:
is as follows:
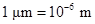
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- Please help me answer the following questions. My answers weren't good enough. Need to know whyy the following chemicals were not used in this experiment related to the melting points and kf values. For lab notebook not a graded assignments.arrow_forwardDraw the arrow pushing reaction mechanism. DO NOT ANSWER IF YOU WONT DRAW IT. Do not use chat gpt.arrow_forwardComplete the following esterification reaction by drawing the structural formula of the product formed. HOH HO i catalyst catalyst OH HO (product has rum flavor) (product has orange flavor)arrow_forward
- The statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant. In each table, there may be one statement that is faise because it contradicts the other three statements. If you find a false statement, check the box next to t Otherwise, check the "no false statements" box under the table. statement false? AG"1 no false statements: statement false? AG-0 0 InK-0 0 K-1 0 AH-TAS no false statements 2arrow_forwardComplete the following esterification reactions by drawing the line formulas of the carboxylic acid and alcohol required to form the ester shown. catalyst catalyst catalyst apricot fragrancearrow_forwardShow the saponification products of the following ester: You don't need to draw in the Na+ cation. catalyst, A catalyst, A catalyst, Aarrow_forward
- What would happen if the carboxylic acid and alcohol groups were on the same molecule? In essence, the molecule reacts with itself. Draw the structure of the products formed in this manner using the reactants below. If two functional groups interact with one another on the same molecule, this is called an “intramolecular" (within one) rather than "intermolecular" (between two or more) attack. OH OH catalyst OH HO catalyst catalyst HO OHarrow_forwardQ3: Write in the starting alkyl bromide used to form the following products. Include any reactants, reagents, and solvents over the reaction arrow. If more than one step is required, denote separate steps by using 1), 2), 3), etc. H OH racemic OH OH 5 racemicarrow_forwardDraw the Lewis structure of the SO3-O(CH3)2 complex shown in the bottom right of slide 2in lecture 3-3 (“Me” means a CH3 group) – include all valence electron pairs and formal charges.From this structure, should the complex be a stable molecule? Explain.arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning



