
Concept explainers
(a)
Interpretation:
The oxidized product of following alcohol when oxidized with
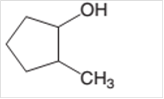
Concept Introduction:
A
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
The oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 14.70P
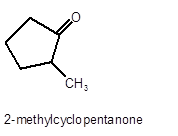
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed:
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or
carboxylic acid as it is overall removal of H atoms. - Primary alcohols are oxidized to
aldehyde which further oxidized to a carboxylic acid. - Secondary alcohols are oxidized to
ketone (R2CO). - Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence, the oxidized of 2-methylcyclopnetanol will form 2-methylcyclopentanone molecule as 2-methylcyclopnetanol is a secondary alcohol.
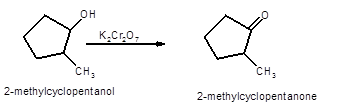
(b)
Interpretation:
The oxidized product of the following alcohol when oxidized with
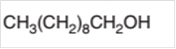
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction, the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactants and products must be separated by an arrow.
The oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 14.70P
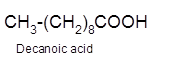
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to carboxylic acid.
- Secondary alcohols are oxidized to ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence, the oxidized of 1-decanol will form decanoic acid molecule as 1-decanol is a primary alcohol.
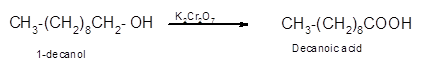
(c)
Interpretation:
The oxidized product of following alcohol when oxidized with
Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactant and products must be separated by an arrow.
Oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 14.70P

Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to carboxylic acid.
- Secondary alcohols are oxidized to ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
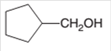
Hence, the oxidized of cyclopentylmethanol will form cyclopentanecarboxylic acid molecule as cyclopentylmethanol is a primary alcohol.
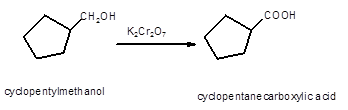
(d)
Interpretation:
The oxidized product of following alcohol when oxidized with

Concept Introduction:
A chemical reaction is the symbolic representation of the conversion of substances to new substances.
In a chemical reaction; the substance which is involved in conversion is said to be reactant whereas the newly formed substance is known as a product. Both reactant and products must be separated by an arrow.
Oxidation reaction is the reaction that involves the addition of O atom in the presence of certain oxidizing agents such as
Answer to Problem 14.70P
2-ethyl-3-pentanol cannot oxidize as it is a tertiary alcohol.
Explanation of Solution
To get the oxidized product of any alcohol, three steps must be followed;
- Locate the C atom in the parent chain that is bonded with −OH group.
- Convert that C atom to carbonyl C atom or carboxylic acid as it is the overall removal of H atoms.
- Primary alcohols are oxidized to aldehyde which further oxidized to the carboxylic acid.
- Secondary alcohol is oxidized to ketone (R2CO).
- Tertiary alcohols are not oxidized as they do not have H atom on the C with the −OH group.
Hence 2-ethyl-3-pentanol cannot oxidize as it is a tertiary alcohol.
Want to see more full solutions like this?
Chapter 14 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- Arrange the following compounds / ions in increasing nucleophilicity (least to most nucleophilic) CH3NH2 CH3C=C: CH3COO 1 2 3 5 Multiple Choice 1 point 1, 2, 3 2, 1, 3 3, 1, 2 2, 3, 1 The other answers are not correct 0000arrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the cured electron-pushing arrows for thw following reaction or mechanistic steps. be sure to account for all bond-breaking and bond making stepsarrow_forwardUsing the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forward
- Synthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forwardShown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forwardHi I need help on the question provided in the image.arrow_forward
- Draw a reasonable mechanism for the following reaction:arrow_forwardDraw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward

 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning




