
Concept explainers
What
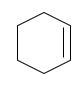
a.
(a)
Interpretation:
The starting material and the reagents needed to prepare following alkyl halide should be determined:
Concept Introduction:
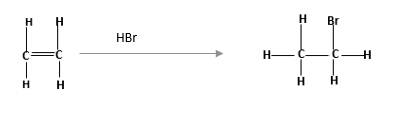
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 59P
Starting alkene-
Reagent -
Explanation of Solution
Unsaturated alkene molecules react with
Refer to the below reaction:
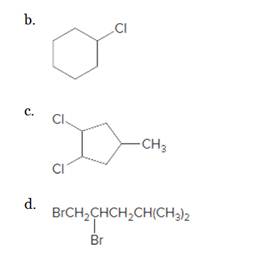
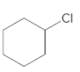
(b)
Interpretation:
The starting material and the reagents needed to prepare following alkyl halide should be determined:
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 59P
Starting alkene −
Reagent -
Explanation of Solution
Unsaturated alkene molecules react with
Refer to the below reaction;
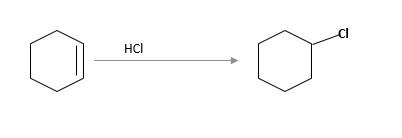
(c)
Interpretation:
The starting material and the reagents needed to prepare following alkyl halide should be determined:
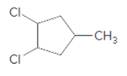
Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 59P
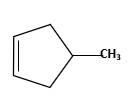 Starting alkene −
Starting alkene −
Reagent -
Explanation of Solution
Unsaturated alkene molecules react with
Refer to the below reaction;
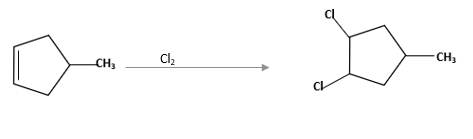
(d)
Interpretation:
The starting material and the reagents needed to prepare following alkyl halide should be determined:

Concept Introduction:
Alkenes are hydrocarbon molecules that consist a carbon-carbon double bond which has the general formula of
Reaction of alkene with
Answer to Problem 59P
Starting alkene −
Reagent -
Explanation of Solution
Unsaturated alkene molecules react with
Refer to the below reaction:
Want to see more full solutions like this?
Chapter 13 Solutions
GENERAL,ORGANIC, & BIOLOGICAL CHEM-ACCES
Additional Science Textbook Solutions
Organic Chemistry (8th Edition)
Organic Chemistry
Fundamentals Of Thermodynamics
Chemistry: Structure and Properties (2nd Edition)
- 2. Provide a complete retrosynthetic analysis and a complete forward synthetic scheme to make the following target molecule from the given starting material. You may use any other reagents necessary. Brarrow_forward146. Use the following data for NH3(g) at 273 K to determine B2p (T) at 273 K. P (bar) 0.10 0.20 0.30 0.40 0.50 0.60 (Z -1)/10-4 1.519 3.038 4.557 6.071 7.583 9.002 0.70 10.551arrow_forward110. Compare the pressures given by (a) the ideal gas law, (b) the van der Waals equation, and (c) the Redlic-Kwong equation for propane at 400 K and p = 10.62 mol dm³. The van der Waals parameters for propane are a = 9.3919 dm6 bar mol-2 and b = 0.090494 dm³ mol−1. The Redlich-Kwong parameters are A = 183.02 dm bar mol-2 and B = 0.062723 dm³ mol-1. The experimental value is 400 bar.arrow_forward
- Research in surface science is carried out using stainless steel ultra-high vacuum chambers with pressures as low as 10-12 torr. How many molecules are there in a 1.00 cm3 volume at this pressure and at a temperature of 300 K? For comparison, calculate the number of molecules in a 1.00 cm3 volume at atmospheric pressure and room temperature. In outer space the pressure is approximately 1.3 x 10-11 Pa and the temperature is approximately 2.7 K (determined using the blackbody radiation of the universe). How many molecules would you expect find in 1.00 cm3 of outer space?arrow_forwardDraw the predominant form of arginine at pH = 7.9. The pKa of the side chain is 12.5. Include proper stereochemistry. H2N OH NH H₂N 'N' છ H pH = 7.9 Select to Drawarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- 142. A mixture of H2(g) and N2(g) has a density of 0.216 g/liter at 300 K and 500 torr. What is the mole fraction composition of the mixture?arrow_forwardOne liter of N2(g) at 2.1 bar and two liters of Ar(g) at 3.4 bar are mixed in a 4.0 liter flask to form an ideal gas mixture. Calculate the value of the final pressure of the mixture if the initial and final temperature of the gases are the same. Repeat this calculation if the initial temperature of the N2(g) and Ar(g) are 304 K and 402 K, respectively, and the final temperature of the mixture is 377 K.arrow_forward10 5 4. These four 'H NMR spectra were recorded from different isomers with molecular formula CsH,CIO. They all contain a carbonyl group. Determine the structure of the different isomers. 0 10 5 0 10 5 10 9 8 7 6 5 4 3. 1 0 9 10 10 66 9 0 10 9 10 5 1 8 7 6 5 3 2 -a 8 7 6 5 1 10 9 8 7 6 5 22 2 1 0 3 2 16 1 0 3 2 1 2 6 0arrow_forward
- Use the expression below to ⚫ calculate its value and report it to the proper number of significant digits (you may need to round your answer). ⚫ calculate the % error (or % relative error or % inherent error) ⚫ calculate the absolute error. (20.54±0.02 × 0.254±0.003) / (3.21±0.05) = Value: % Error: Absolute error: ± | % (only 1 significant digit) (only 1 significant digit)arrow_forwardIn each case (more ductile, more brittle, more tough or resistant), indicate which parameter has a larger value. parameter Elastic limit Tensile strength more ductile Strain at break Strength Elastic modulus more fragile more tough or resistantarrow_forwardNonearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





