
Concept explainers
(a)
Interpretation: To determine the net gain or net loss in triphosphates (ATP, UTP, etc.) in glycolysis.
Concept introduction: Glycolysis is the
Triphosphate nucleotides provide energy to carry out the metabolic processes in the living cells. Adenosine triphosphate (ATP), Cytidine triphosphate (CTP), Uridine triphosphate (UTP), and Guanosine triphosphate (GTP) are examples of triphosphate nucleotides.
(b)
Interpretation: To determine the net gain or net loss in triphosphates (ATP, UTP, etc.) in glycogenolysis.
Concept introduction: Glycogenolysis is the metabolic pathway that converts glycogen to
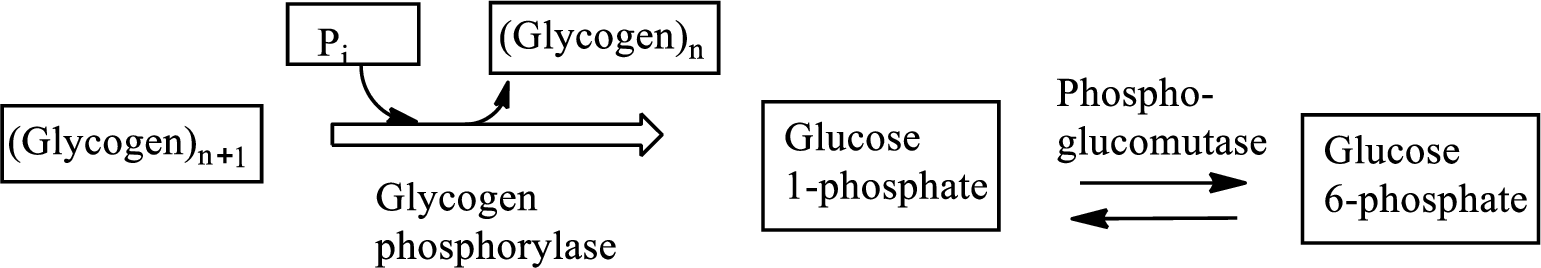
Triphosphate nucleotides provide energy to carry out the metabolic processes in the living cells. Adenosine triphosphate (ATP), Cytidine triphosphate (CTP), Uridine triphosphate (UTP), and Guanosine triphosphate (GTP) are examples of triphosphate nucleotides.
(c)
Interpretation: To determine the net gain or net loss in triphosphates (ATP, UTP, etc.) when glycogen glucose unit is converted to glucose.
Concept introduction: Glucose is a monosaccharide with the molecular formula
Triphosphate nucleotides provide energy to carry out the metabolic processes in the living cells. Adenosine triphosphate (ATP), Cytidine triphosphate (CTP), Uridine triphosphate (UTP), and Guanosine triphosphate (GTP) are examples of triphosphate nucleotides.
(d)
Interpretation: To determine the net gain or net loss in triphosphates (ATP, UTP, etc.) when glycogen glucose unit is converted to lactate.
Concept introduction: Glycogenolysis is the metabolic pathway that converts glycogen to
Lactate dehydrogenase enzymes convert pyruvate to lactate under anaerobic conditions in the human body. This anaerobic reduction of pyruvate to form lactate by enzymes is called lactate fermentation.
Triphosphate nucleotides provide energy to carry out the metabolic processes in the living cells. Adenosine triphosphate (ATP), Cytidine triphosphate (CTP), Uridine triphosphate (UTP), and Guanosine triphosphate (GTP) are examples of triphosphate nucleotides.
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Organic And Biological Chemistry
- Which of the following reagents best achieves the reaction shown below? Why is the correct answer B? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardWhat is the product of the following reaction sequence? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardPls help ASAParrow_forward
- The reaction of phenylmagnesium bromide (C6H5MgBr) with propanal (CH3CH2CHO)3 followed by hydrolysis yields. A. 2-phenyl-1-propanol B. 1-phenyl-1propanol C. 3-phenyl-2-propanol D. 3-phenyl-1-propanol Why is the correct answer B? Please explain what is happening. Please include a detailed explanation and/or a drawing of steps needed to understand the reaction or question.arrow_forwardWhat is the product of the reaction sequence below? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question. The part that is under the pen in the image is (1) CH3CHO (2) H3O+arrow_forwardWhat is the missing reactant in this organic reaction? R+ OH HD CH3-CH2-CH-CH3 H A CH3 CH3-CH2-C-O-CH-CH2-CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No answer Click anywhere to draw the first atom of your structure. ☐ : Jm +arrow_forward
- Draw the major product of the following E2 reaction. Make sure you pay attention to REGIOCHEMISTRY and STEREOCHEMISTRY. Explain why this product is formed using 10 words or less for each. (a) NaH Br acetone TSO, NaH (b) acetonearrow_forward2. Circle the compound that will react SLOWER in an E2 reaction. To get credit for this question, you must EXPLAIN how you got your answer using STRUCTURES and WORDS. Br ** Br...arrow_forward8. 2 20 00 Draw ALL of the possible products for the following reaction CIRCLE the MAJOR product NaOMe MeOHarrow_forward
- NAME: 1. Draw the major product of the following E2 reaction. Make sure you pay attention to REGIOCHEMISTRY and STEREOCHEMISTRY. To get credit for this question, you must EXPLAIN how you got your answer using STRUCTURES and WORDS. Br NaOCH3 acetone F2 reaction To get credit for thisarrow_forward3. Reactions! Fill in the information missing below. Make sure to pay attention to REGIOCHEMISTRY and STEREOCHEMISTRY. Br2 CH3OH + 4. Mechanism! Show the complete arrow pushing mechanism, including all steps and intermediates for the following reactions. To get credit for this, you MUST show how ALL bonds are broken and formed, using arrows to show the movement of electrons. H3O+ HOarrow_forwardPlease provide a synthesis for the Ester using proponoic acid, thank you!arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning  Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning




