
Concept explainers
(a)
Interpretation: To indicate whether lactate is involved in (1) the pentose phosphate pathway, (2) the Cori cycle, (3) glycolysis, or (4) lactate fermentation.
Concept introduction: The pentose phosphate pathway is defined as the metabolic pathway in which NADPH,
Glucose is converted to pyruvate by glycolysis metabolic pathway; pyruvate is further converted to lactate in the skeletal muscle cells by anaerobic reactions. The lactate is diffused into the bloodstream, by which it is transported to the liver. Lactate is reconverted to pyruvate. Gluconeogenesis metabolic pathway uses this pyruvate to synthesize glucose in the liver cells. Glucose is diffused into the bloodstream and is transported back to the active skeletal muscle cells. This cycle is known as the Cori cycle.
Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
Fermentation is defined as the biochemical anaerobic process by which NADH is oxidized to
The structure of lactate is as follows:
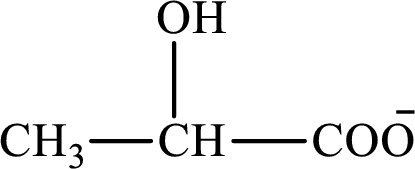
(a)
Answer to Problem 13.105EP
Lactate is associated with (2) the Cori cycle and (4) the lactate fermentation.
Explanation of Solution
An overview of the Cori cycle is as follows:
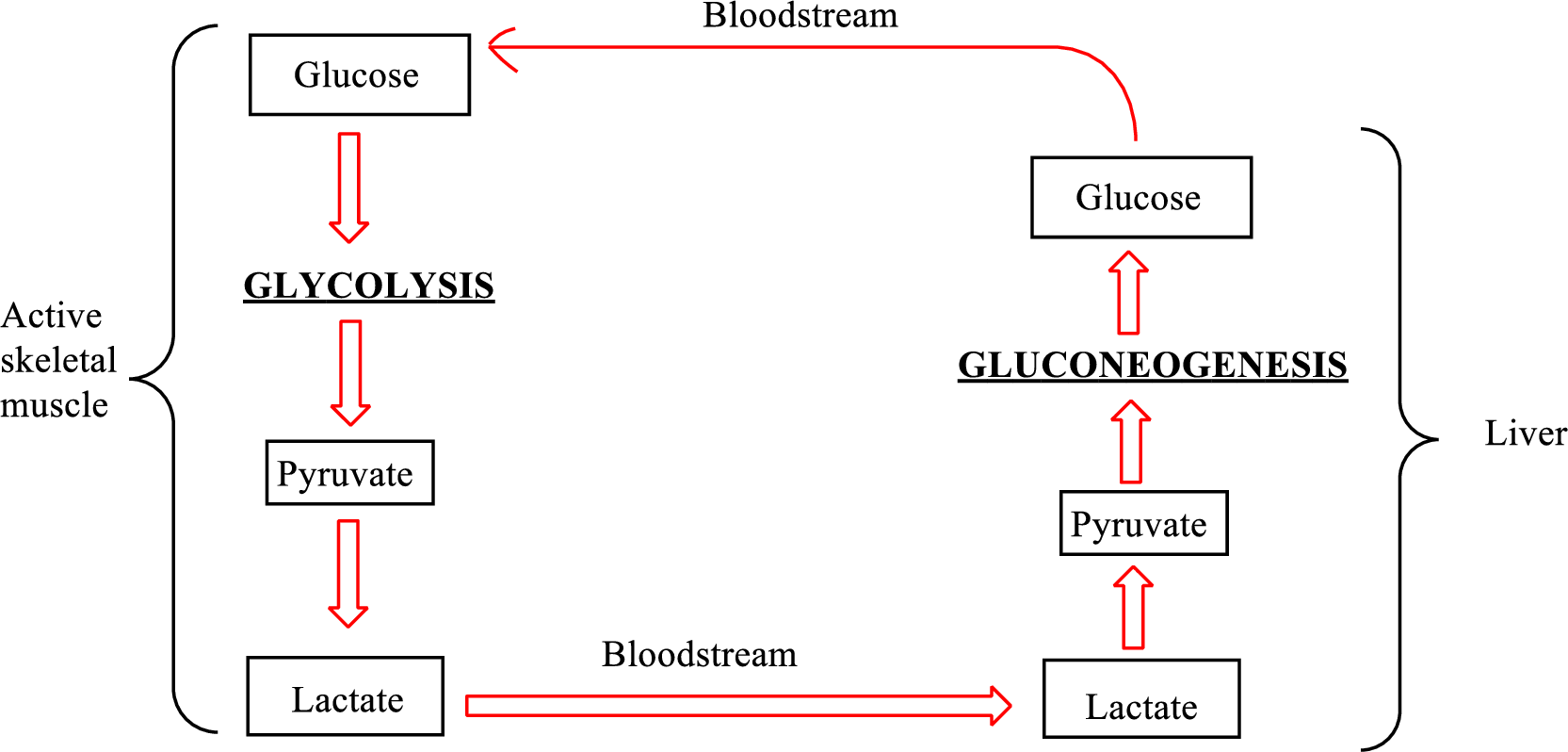
Lactate is converted to pyruvate in the liver. Therefore, lactate is associated with the Cori cycle.
Pyruvate is converted to lactate under oxygen-poor conditions by lactate dehydrogenase enzymes in the human body. This anaerobic reduction of pyruvate to form lactate by enzymes is called lactate fermentation. The
Therefore, lactate is associated with the Cori cycle and the lactate fermentation.
(b)
Interpretation: To indicate whether
Concept introduction: The pentose phosphate pathway is defined as the metabolic pathway in which NADPH,
Glucose is converted to pyruvate by glycolysis metabolic pathway; pyruvate is further converted to lactate in the skeletal muscle cells by anaerobic reactions. The lactate is diffused into the bloodstream, by which it is transported to the liver. Lactate is reconverted to pyruvate. Gluconeogenesis metabolic pathway uses this pyruvate to synthesize glucose in the liver cells. Glucose is diffused into the bloodstream and is transported back to the active skeletal muscle cells. This cycle is known as the Cori cycle.
Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
Pyruvate is converted to lactate under oxygen-poor conditions by lactate dehydrogenase enzymes in the human body. This anaerobic reduction of pyruvate to form lactate by enzymes is called lactate fermentation.
Nicotinamide adenine dinucleotide is associated with the
(b)
Answer to Problem 13.105EP
Explanation of Solution
An overview of the Cori cycle is as follows:
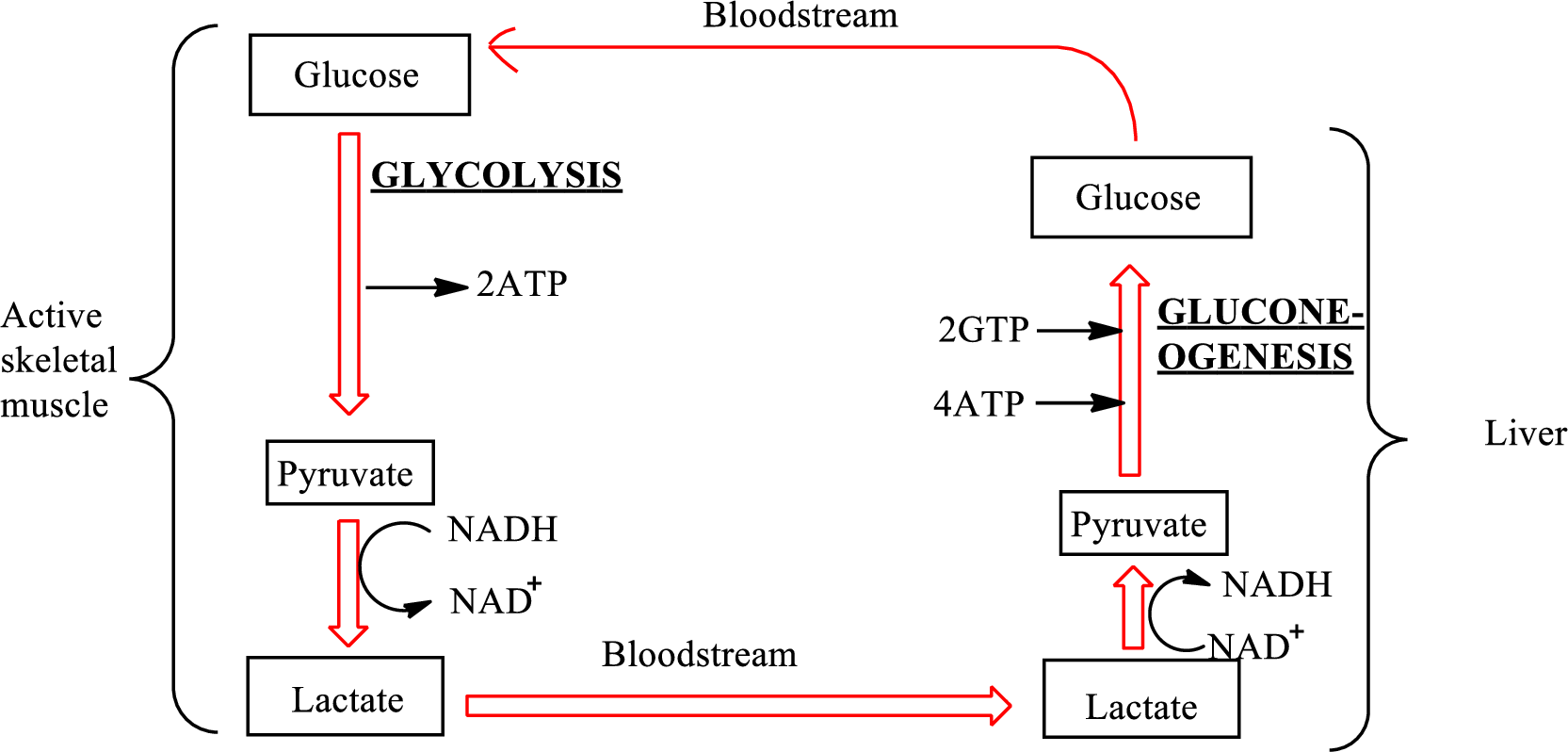
In the Cori cycle,
Pyruvate is converted to lactate under oxygen-poor conditions by lactate dehydrogenase enzymes in the human body. This anaerobic reduction of pyruvate to form lactate by enzymes is called lactate fermentation. The chemical reaction for the formation of lactate is as follows:
In the lactate fermentation, NADH is oxidized to
The net overall equation for the glycolysis process is as follows:
(c)
Interpretation: To indicate whether
Concept introduction: The pentose phosphate pathway is defined as the metabolic pathway in which NADPH,
Glucose is converted to pyruvate by glycolysis metabolic pathway; pyruvate is further converted to lactate in the skeletal muscle cells by anaerobic reactions. The lactate is diffused into the bloodstream, by which it is transported to the liver. Lactate is reconverted to pyruvate. Gluconeogenesis metabolic pathway uses this pyruvate to synthesize glucose in the liver cells. Glucose is diffused into the bloodstream and is transported back to the active skeletal muscle cells. This cycle is known as the Cori cycle.
Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
Pyruvate is converted to lactate under oxygen-poor conditions by lactate dehydrogenase enzymes in the human body. This anaerobic reduction of pyruvate to form lactate by enzymes is called lactate fermentation.
(c)
Answer to Problem 13.105EP
Explanation of Solution
The first stage of the pentose phosphate pathway is the oxidative stage. In the oxidative stage (involves three steps) of the pentose phosphate pathway,
The general equation for the entire pentose phosphate pathway is as follows:
Therefore,
The net overall equation for the glycolysis process is as follows:
Glucose enters the glycolysis metabolic pathway in the form of
(d)
Interpretation: To indicate whether
Concept introduction: The pentose phosphate pathway is defined as the metabolic pathway in which NADPH,
Glucose is converted to pyruvate by glycolysis metabolic pathway; pyruvate is further converted to lactate in the skeletal muscle cells by anaerobic reactions. The lactate is diffused into the bloodstream, by which it is transported to the liver. Lactate is reconverted to pyruvate. Gluconeogenesis metabolic pathway uses this pyruvate to synthesize glucose in the liver cells. Glucose is diffused into the bloodstream and is transported back to the active skeletal muscle cells. This cycle is known as the Cori cycle.
Glycolysis is the metabolic pathway that breaks down a glucose molecule and converts it into two pyruvate molecules along with the production of two ATP molecules and NADH coenzymes.
Pyruvate is converted to lactate under oxygen-poor conditions by lactate dehydrogenase enzymes in the human body. This anaerobic reduction of pyruvate to form lactate by enzymes is called lactate fermentation.
(d)
Answer to Problem 13.105EP
Explanation of Solution
The first stage of the pentose phosphate pathway is the oxidative stage. In the oxidative stage (involves three steps) of the pentose phosphate pathway,
The first step of the second stage i.e. the non-oxidative stage of the pentose phosphate pathway is the isomerization of
Want to see more full solutions like this?
Chapter 13 Solutions
Organic And Biological Chemistry
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





