
Concept explainers
(a)
Interpretation: To identify
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions. The common fates of pyruvate are as follows:
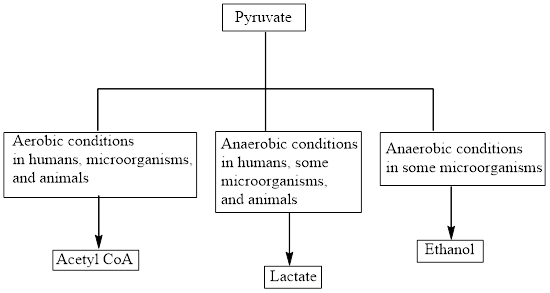
Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
(a)
Answer to Problem 13.45EP
Carbon dioxide
Explanation of Solution
Reason for correct choice:
Under aerobic conditions, pyruvate is converted to
The process of ethanol fermentation takes place in two steps. In step 1, the pyruvate molecule is converted to acetaldehyde by pyruvate decarboxylase enzymes. Carbon dioxide molecule is produced in this step. In step 2, acetaldehyde is reduced to ethanol by alcohol dehydrogenase enzymes. The ethanol fermentation equation is as follows:
Therefore,
Reason for incorrect choice:
The reaction equation for lactate fermentation is as follows:
(b)
Interpretation: To identify NADH is a reactant in which the fate of pyruvate-
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions.
The common fates of pyruvate are as follows:

Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
Nicotinamide adenine dinucleotide is associated with the
A reactant is defined as the substance that is initially present in the
(b)
Answer to Problem 13.45EP
NADH is encountered as a reactant in the lactate and ethanol production from pyruvate.
Explanation of Solution
Reason for correct choice:
In the absence of oxygen, pyruvate is converted to lactate by lactate dehydrogenase enzymes in the human body. In this reaction, NADH is oxidized to
Ethanol fermentation process occurs in some microorganisms (for example yeast) under the anaerobic conditions. The ethanol fermentation equation is as follows:
Therefore, NADH is encountered as a reactant in the lactate and ethanol production from pyruvate.
Reason for incorrect choice:
The reaction equation for the conversion of pyruvate to
Therefore, NADH is formed along with
(c)
Interpretation: To identify
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions. The common fates of pyruvate are as follows:

Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
Nicotinamide adenine dinucleotide is associated with the redox reactions in metabolism. Its reduced form is NADH and oxidized form is
A reactant is defined as the substance that is initially present in the chemical reaction and gets consumed to form a new substance.
(c)
Answer to Problem 13.45EP
In the production of
Explanation of Solution
Reason for correct choice:
The reaction equation for the conversion of pyruvate to
Therefore,
Reason for incorrect choice:
The reaction equation for the conversion of pyruvate to lactate is as follows:
Ethanol fermentation process occurs in some microorganisms (for example yeast) under the anaerobic conditions. The ethanol fermentation equation is as follows:
Therefore,
(d)
Interpretation: To identify the end product is a
Concept introduction: Pyruvate is the end product in the glycolysis. The production of the fate of pyruvate varies with the nature of the organism and the cellular conditions. The common fates of pyruvate are as follows:

Aerobic reactions need oxygen while anaerobic reactions don’t need oxygen. Pyruvate forms
Pyruvate
(d)
Answer to Problem 13.45EP
In the absence of oxygen, pyruvate is converted to
Explanation of Solution
Reason for correct choice:
In the absence of oxygen, pyruvate is converted to lactate by lactate dehydrogenase enzymes in the human body. This anaerobic reduction is called lactate fermentation. The chemical reaction for the formation of lactate is as follows:

Lactate contains three carbon atoms. Therefore, lactate is a
Reason for incorrect choice:
In the ethanol fermentation process, pyruvate is converted to ethanol and carbon dioxide by enzymes under the anaerobic conditions. The ethanol fermentation equation is as follows:
Ethanol
Pyruvate is converted to
Acetyl group
Want to see more full solutions like this?
Chapter 13 Solutions
Organic And Biological Chemistry
- All of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forward
- Name the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forward
- Reaction Fill-ins Part 2! Predict the product(s) OR starting material of the following reactions. Remember, Hydride shifts are possible if/when a more stable carbocation can exist (depending on reaction mechanism)! Put your answers in the indicated boxes d. d. ง HCIarrow_forwardA cylinder contains 12 L of water vapour at 150˚C and 5 atm. The temperature of the water vapour is raised to 175˚C, and the volume of the cylinder is reduced to 8.5 L. What is the final pressure of the gas in atmospheres? assume that the gas is idealarrow_forwardOn the next page is an LC separation of the parabens found in baby wash. Parabens are suspected in a link to breast cancer therefore an accurate way to quantitate them is desired. a. In the chromatogram, estimate k' for ethyl paraben. Clearly indicate what values you used for all the terms in your calculation. b. Is this a "good" value for a capacity factor? Explain. c. What is the resolution between n-Propyl paraben and n-Butyl paraben? Again, indicate clearly what values you used in your calculation. MAU | Methyl paraben 40 20 0 -2 Ethyl paraben n-Propyl paraben n-Butyl paraben App ID 22925 6 8 minarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co




