
Concept explainers
(a)
Interpretation: To identify the number of steps in glycolysis that produce ATP.
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:
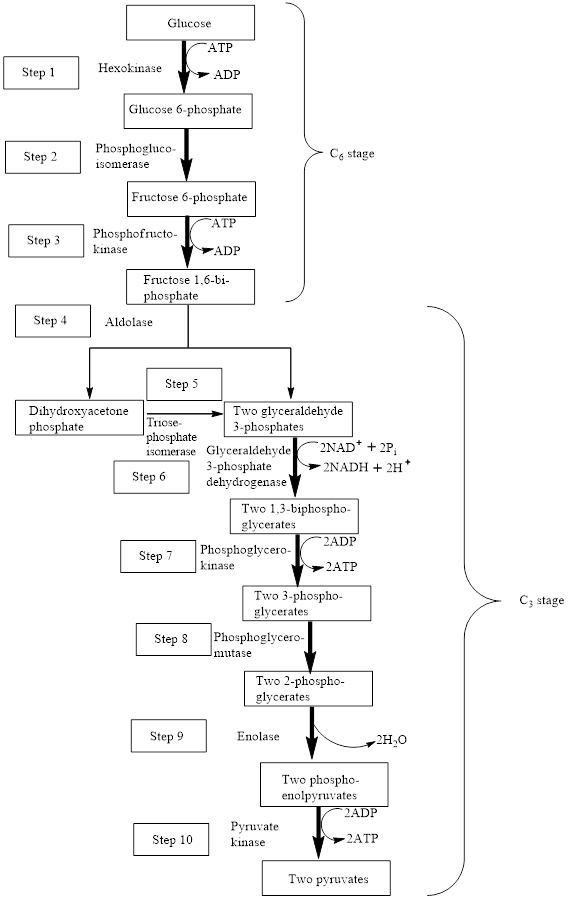
Adenosine triphosphate (ATP) is a molecule that is defined as the energy currency of life and provides energy to carry out the metabolic processes in the living cells. It is converted either to adenosine monophosphate (AMP) or to adenosine diphosphate (ADP) after the consumption in the metabolic processes.
(a)
Answer to Problem 13.17EP
In glycolysis, two steps produce ATP.
Explanation of Solution
In step 7 and step 10, two ATP molecules are produced in each step. Hence, in the glycolysis pathway, four ATP molecules are produced is two steps.
(b)
Interpretation: To identify the number of steps in glycolysis that involve phosphorylation.
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:

In the phosphorylation reaction, the molecule is attached to the phosphoryl group. The transfer of a phosphoryl group
(b)
Answer to Problem 13.17EP
Phosphorylation is involved in five steps.
Explanation of Solution
The first step in the glycolysis process is the phosphorylation of glucose using ATP. Glucose is converted to
In the third step,
In step 6,
Therefore, step 6 proceeds through the oxidation and phosphorylation reaction using
The seventh step in the glycolysis process is the phosphorylation of
Step 10 is the final step of the glycolysis process. In step 10, phosphoenolpyruvate is converted to pyruvate using ADP, thus, ADP is converted to ATP. Pyruvate kinase enzymes are involved in this reaction. The type of reaction is phosphorylation. The word equation for the phosphorylation reaction is as follows:
Therefore, step 1, step 2, step 6, step 7 and step 10 involved the phosphorylation reaction.
(c)
Interpretation: To identify the number of steps in glycolysis that involve
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:

A reactant is defined as the substance that is initially present in the
Nicotinamide adenine dinucleotide is associated with the
(c)
Answer to Problem 13.17EP
One step involves
Explanation of Solution
In step 6,
Therefore,
(d)
Interpretation: To identify the number of steps in glycolysis that involve a compound with a high-energy bond as a reactant.
Concept introduction: In the glycolysis metabolic pathway, a glucose molecule breaks down into two pyruvate molecules. Two ATP molecules and NADH coenzymes are produced along with pyruvate.
The block diagram to represent an overview of glycolysis is as follows:

A reactant is defined as the substance that is initially present in the chemical reaction and gets consumed to form a new substance.
High energy compounds are those compounds that release a large amount of energy upon hydrolysis. These compounds consist of highly strained bonds that are responsible for the release of a high amount of energy. The compounds containing a phosphate group are examples of high energy compounds.
A high-energy phosphate group is formed when a phosphate group is attached to a carbon atom participating in carbon-oxygen or carbon-carbon double bond.
(d)
Answer to Problem 13.17EP
Two steps involve a compound with a high-energy bond as a reactant.
Explanation of Solution
The seventh step in the glycolysis process is the phosphorylation of
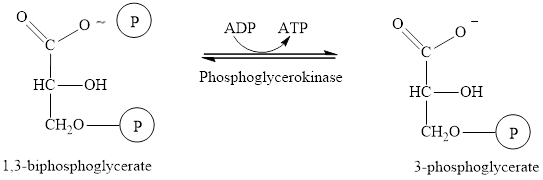
Here,  denotes the
denotes the
In step 10, phosphoenolpyruvate is converted to pyruvate using ADP, thus, ADP is converted to ATP. Pyruvate kinase enzymes are involved in this reaction. Phosphoenolpyruvate is a compound with a high-energy phosphate group because the phosphate group is attached to that carbon atom which is involved in carbon-carbon double bond. The chemical reaction is as follows:

Therefore, in step 10, a high-energy bond is encountered as a reactant.
Want to see more full solutions like this?
Chapter 13 Solutions
Organic And Biological Chemistry
- Predict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward
- What is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forward
- Predict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forwardPredict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forward
- Predict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forwardYou are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





