
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 13.33P
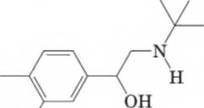
13-33 Following is the structural formula of albuterol
(Proventil), one of the most widely used inhalation bronchodilators.
HO Z
- Name the
functional groups present in albuterol - Draw a structural formula for the product formed when albuterol is treated with one equivalent of aqueous sodium hydroxide.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please correct answer and don't use hand rating
Please correct answer and don't use hand rating
The SN 1 mechanism starts with the rate-determining step which is the dissociation of the alkyl halide into a carbocation and a halide ion. The next step is
the rapid reaction of the carbocation intermediate with the nucleophile; this step completes the nucleophilic substitution stage. The step that follows the
nucleophilic substitution is a fast acid-base reaction. The nucleophile now acts as a base to remove the proton from the oxonium ion from the previous
step, to give the observed product. Draw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all nonzero formal
charges.
Cl:
Add/Remove step
G
Click and drag to start
drawing a structure.
Chapter 13 Solutions
Introduction to General, Organic and Biochemistry
Ch. 13.2 - Prob. 13.1PCh. 13 - Answer true or false. Alkenes, alkynes, and arenes...Ch. 13 - 13-3 What is the difference in structure between a...Ch. 13 - 13-4 Define aromatic compound.Ch. 13 - 13-5 Why are alkenes, alkynes, and aromatic...Ch. 13 - 13-B Do aromatic rings have double bonds? Are they...Ch. 13 - 13-7 Can an aromatic compound be a saturated...Ch. 13 - Draw at least two structural formulas for each of...Ch. 13 - 13-9 Write a structural formula and the name for...Ch. 13 - 13-10 Account for the fact that the six-membered...
Ch. 13 - 13-11 Explain why the compound 1,4-dichlorobenzene...Ch. 13 - 13-12 One analogy often used to explain the...Ch. 13 - 13-13 Answer true or false. A phenyl group has the...Ch. 13 - Prob. 13.14PCh. 13 - 13-15 Draw structural formulas for these compounds...Ch. 13 - 13-16 We say that naphthalene, anthracene,...Ch. 13 - 13-17 Following is the structural formula of...Ch. 13 - 13-18 Answer true or false. Benzene does not...Ch. 13 - 13-19 Suppose you have unlabeled bottles of...Ch. 13 - 13-20 Three products with the molecular formula...Ch. 13 - 13-21 The reaction of bromine with toluene in the...Ch. 13 - 13-22 What reagents and/or catalysts are necessary...Ch. 13 - 13-23 What reagents and/or catalysts are necessary...Ch. 13 - Prob. 13.24PCh. 13 - 13-25 Answer true or false. (a) Phenols and...Ch. 13 - 13-26 Both phenol and cyclohexanol are only...Ch. 13 - 13-27 Define autoxidation.Ch. 13 - 13*28 Autoxidation is described as a radical-chain...Ch. 13 - 13-29 Show that if you add Steps 2a and 2b of the...Ch. 13 - 13-30 How does vitamin E function as an...Ch. 13 - 13-31 What structural features are common to...Ch. 13 - 13*32 Black-and-white photography is a commercial...Ch. 13 - 13-33 Following is the structural formula of...Ch. 13 - 13-34 (Chemical Connections 13A) From what parts...Ch. 13 - Prob. 13.35PCh. 13 - 13-36 (Chemical Connections 13A, Would you expect...Ch. 13 - Prob. 13.37PCh. 13 - 13-38 (Chemical Connections 13A) What is meant by...Ch. 13 - 13-39 (Chemical Connections 13B) What is a...Ch. 13 - 13-40 (Chemical Connections 130 In the absence of...Ch. 13 - Prob. 13.41PCh. 13 - 13-42 (Chemical Connections 13E) What are the...Ch. 13 - 13-43 (Chemical Connections 13E) Which features of...Ch. 13 - 13-44 (Chemical Connections 13E) What color would...Ch. 13 - Prob. 13.45PCh. 13 - Prob. 13.46PCh. 13 - Prob. 13.47PCh. 13 - 13-48 (Chemical Connections 13F, How many...Ch. 13 - 13-49 (Chemical Connections 13F) In what ways is...Ch. 13 - 13*50 The structure for naphthalene given in...Ch. 13 - 13-51 Draw structural formulas for these...Ch. 13 - 13-52 2,6-Di-/ezY-butyl-4-methylphenol (BHT,...Ch. 13 - 13-53 Write the structural formula for the product...Ch. 13 - 13-54 Styrene reacts with bromine to give a...Ch. 13 - 13-55 When toluene is treated with Br, in the...Ch. 13 - 13-56 Four alternatives to the structure of...Ch. 13 - 13-57 Benzene, as we have seen in this chapter, is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please correct answer and don't use hand ratingarrow_forwardA monochromatic light with a wavelength of 2.5x10-7m strikes a grating containing 10,000 slits/cm. Determine the angular positions of the second-order bright line.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Us the reaction conditions provided and follow the curved arrow to draw the resulting structure(s). Include all lone pairs and charges as appropriate. H :I H 0arrow_forward
- Please correct answer and don't use hand ratingarrow_forwardNonearrow_forwardYou have started a patient on a new drug. Each dose introduces 40 pg/mL of drug after redistribution and prior to elimination. This drug is administered at 24 h intervals and has a half life of 24 h. What will the concentration of drug be after each of the first six doses? Show your work a. What is the concentration after the fourth dose? in pg/mL b. What is the concentration after the fifth dose? in pg/mL c. What is the concentration after the sixth dose? in pg/mLarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY