
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 13.37P
Interpretation Introduction
Interpretation:
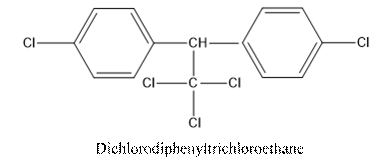
The structural formula of DDE (Dichlorodiphenyldichloroethylene) should be drawn.
Concept Introduction:
The common name of DDT is dichlorodiphenyltrichloroethane.
DDT is an insecticide. It has two
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
V
Highlight all of the carbon atoms that have at least one beta (B) hydrogen, using red for one ẞ hydrogen, blue for two ẞ hydrogens, and green for three ẞ
hydrogens.
If none of the carbon atoms have ẞ hydrogens, check the box underneath the molecule.
ED
X
None of the carbon atoms have ẞ hydrogens.
Explanation
esc
2
Check
*
F1
F2
1
2
80
# 3
Q
W
tab
A
caps lock
shift
fn
control
F3
N
S
option
O
694
$
F4
F5
F6
005
%
E
R
D
F
LL
6
olo
18
Ar
B
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
A
DII
F7
F8
87
&
*
8
T
Y
U
G
H
4
F9
F10
(
9
0
E
F11
F12
உ
J
K
L
+ ||
X
C
V
B
N
M
H
H
command
option
command
Consider the reaction below and answer the following questions.
Part 1 of 4
Br
NaOCH2CH3
Identify the mechanisms involved. Check all that apply.
SN 1
SN 2
E1
E2
None of the above
Part 2 of 4
Skip Part
Check
esc
F1
F2
lock
1
2
Q
W
A
S
#3
80
F3
F4
F5
F6
Save For
© 2025 McGraw Hill LLC. All Rights Reserved. Terms
ˇˇ
%
&
4
5
6
89
7
IK
A
分
བ
F7
F8
F9
F
*
E
R
T
Y
U
8
9
D
F
G
H
K
V
B
N
M
0
O
What kind of holes are not generated when solid-state particles adopt a close packing pattern?
Group of answer choices
tetrahedral
cubic
octahedral
None of the other choices are correct
Chapter 13 Solutions
Introduction to General, Organic and Biochemistry
Ch. 13.2 - Prob. 13.1PCh. 13 - Answer true or false. Alkenes, alkynes, and arenes...Ch. 13 - 13-3 What is the difference in structure between a...Ch. 13 - 13-4 Define aromatic compound.Ch. 13 - 13-5 Why are alkenes, alkynes, and aromatic...Ch. 13 - 13-B Do aromatic rings have double bonds? Are they...Ch. 13 - 13-7 Can an aromatic compound be a saturated...Ch. 13 - Draw at least two structural formulas for each of...Ch. 13 - 13-9 Write a structural formula and the name for...Ch. 13 - 13-10 Account for the fact that the six-membered...
Ch. 13 - 13-11 Explain why the compound 1,4-dichlorobenzene...Ch. 13 - 13-12 One analogy often used to explain the...Ch. 13 - 13-13 Answer true or false. A phenyl group has the...Ch. 13 - Prob. 13.14PCh. 13 - 13-15 Draw structural formulas for these compounds...Ch. 13 - 13-16 We say that naphthalene, anthracene,...Ch. 13 - 13-17 Following is the structural formula of...Ch. 13 - 13-18 Answer true or false. Benzene does not...Ch. 13 - 13-19 Suppose you have unlabeled bottles of...Ch. 13 - 13-20 Three products with the molecular formula...Ch. 13 - 13-21 The reaction of bromine with toluene in the...Ch. 13 - 13-22 What reagents and/or catalysts are necessary...Ch. 13 - 13-23 What reagents and/or catalysts are necessary...Ch. 13 - Prob. 13.24PCh. 13 - 13-25 Answer true or false. (a) Phenols and...Ch. 13 - 13-26 Both phenol and cyclohexanol are only...Ch. 13 - 13-27 Define autoxidation.Ch. 13 - 13*28 Autoxidation is described as a radical-chain...Ch. 13 - 13-29 Show that if you add Steps 2a and 2b of the...Ch. 13 - 13-30 How does vitamin E function as an...Ch. 13 - 13-31 What structural features are common to...Ch. 13 - 13*32 Black-and-white photography is a commercial...Ch. 13 - 13-33 Following is the structural formula of...Ch. 13 - 13-34 (Chemical Connections 13A) From what parts...Ch. 13 - Prob. 13.35PCh. 13 - 13-36 (Chemical Connections 13A, Would you expect...Ch. 13 - Prob. 13.37PCh. 13 - 13-38 (Chemical Connections 13A) What is meant by...Ch. 13 - 13-39 (Chemical Connections 13B) What is a...Ch. 13 - 13-40 (Chemical Connections 130 In the absence of...Ch. 13 - Prob. 13.41PCh. 13 - 13-42 (Chemical Connections 13E) What are the...Ch. 13 - 13-43 (Chemical Connections 13E) Which features of...Ch. 13 - 13-44 (Chemical Connections 13E) What color would...Ch. 13 - Prob. 13.45PCh. 13 - Prob. 13.46PCh. 13 - Prob. 13.47PCh. 13 - 13-48 (Chemical Connections 13F, How many...Ch. 13 - 13-49 (Chemical Connections 13F) In what ways is...Ch. 13 - 13*50 The structure for naphthalene given in...Ch. 13 - 13-51 Draw structural formulas for these...Ch. 13 - 13-52 2,6-Di-/ezY-butyl-4-methylphenol (BHT,...Ch. 13 - 13-53 Write the structural formula for the product...Ch. 13 - 13-54 Styrene reacts with bromine to give a...Ch. 13 - 13-55 When toluene is treated with Br, in the...Ch. 13 - 13-56 Four alternatives to the structure of...Ch. 13 - 13-57 Benzene, as we have seen in this chapter, is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. 田 Major Product: Check ☐ + I Na OH esc F1 F2 2 1 @ 2 Q W tab A caps lock S #3 80 F3 69 4 σ F4 % 95 S Click and drag to sta drawing a structure mm Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use GO DII F5 F6 F7 F8 F9 F10 6 CO 89 & 7 LU E R T Y U 8* 9 0 D F G H J K L Z X C V B N M 36arrow_forwardProblem 7 of 10 Draw the major product of this reaction. Ignore inorganic byproducts. S' S 1. BuLi 2. ethylene oxide (C2H4O) Select to Draw a Submitarrow_forwardFeedback (4/10) 30% Retry Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the reactant and missing intermediates involved in this reaction. Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. Incorrect, 6 attempts remaining :0: Draw the Reactant H H3CO H- HIO: Ö-CH3 CH3OH2* protonation H. a H (+) H Ο CH3OH2 O: H3C protonation CH3OH deprotonation > CH3OH nucleophilic addition H. HO 0:0 Draw Intermediate a Xarrow_forward
- Can I please get the blank spaces answered/answers?arrow_forward1. Identify the following alkenes as E or Z NH₂ Br 2. Draw the structures based on the IUPAC names (3R,4R)-3-bromo-4-fluoro- 1-hexene (Z)-4-bromo-2-iodo-3-ethyl- 3-heptene تر 3. For the following, predict all possible elimination product(s) and circle the major product. HO H₂SO4 Heat 80 F4 OH H2SO4 Heat 어요 F5 F6 1 A DII 4 F7 F8 F9 % & 5 6 7 * ∞ 8 BAB 3 E R T Y U 9 F D G H J K O A F11 F10arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. ○ O 1. H₂O, pyridine 2. neutralizing work-up a N W X 人 Parrow_forward
- ✓ Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. tab OH CI 0 Br xx Br None of these molecules have a total of five ẞ hydrogens. esc Explanation Check caps lock shift 1 fn control 02 F2 W Q A N #3 S 80 F3 E $ t 01 205 % 5 F5 & 7 © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility FT * 8 R T Y U כ F6 9 FIG F11 F D G H J K L C X V B < N M H option command P H + F12 commandarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts and the carboxylic acid side product. O 1. CHзMgBr (excess) 2. H₂O ✓ W X 人arrow_forwardIf cyclopentyl acetaldehyde reacts with NaOH, state the product (formula).arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. N S S HgCl2, H2SO4 く 8 W X Parrow_forwardtab esc く Drawing the After running various experiments, you determine that the mechanism for the following reaction occurs in a step-wise fashion. Br + OH + Using this information, draw the correct mechanism in the space below. 1 Explanation Check F2 F1 @2 Q W A os lock control option T S # 3 80 F3 Br $ 4 0105 % OH2 + Br Add/Remove step X C F5 F6 6 R E T Y 29 & 7 F D G H Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce A F7 DII F8 C Ո 8 * 9 4 F10 F C J K L C V Z X B N M H command P ge Coarrow_forwardIndicate compound A that must react with ethylbenzene to obtain 4-ethylbenzene-1-sulfonic acid. 3-bromo-4-ethylbenzene-1-sulfonic acid.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

ENVIRONMENTAL POLLUTION; Author: 7activestudio;https://www.youtube.com/watch?v=oxtMFmDTv3Q;License: Standard YouTube License, CC-BY