
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 32P
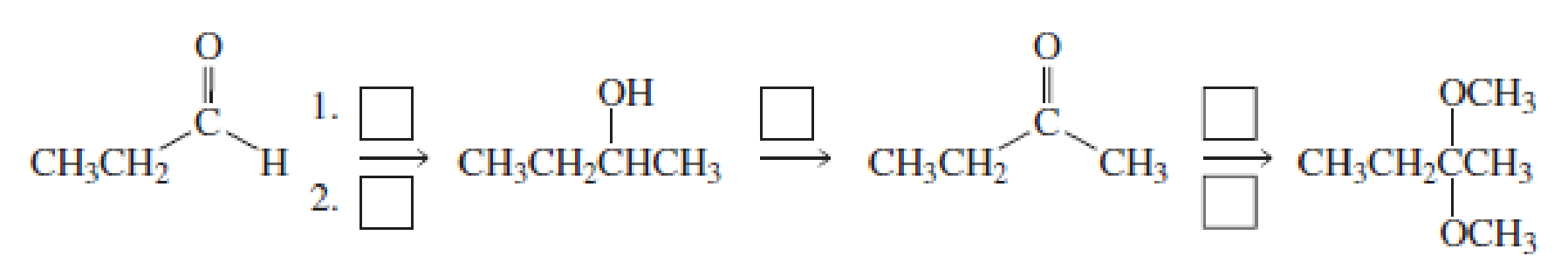
Fill in the boxes:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.
What is the reason of the following(use equations if possible)
a.) In MO preperation through diazotization: Addition of sodium nitrite in acidfied solution in order to form diazonium salt
b.) in MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at low pH
c.) In MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at pH 4.5
d.) Avoiding not cooling down the reaction mixture when preparing the diazonium salt
e.) Cbvc
A 0.552-g sample of an unknown acid was dissolved in water to a total volume of 20.0 mL. This sample was titrated with 0.1103 M KOH. The equivalence point occurred at 29.42 mL base added. The pH of the solution at 10.0 mL base added was 3.72.
Determine the molar mass of the acid.
Determine the Ka of the acid.
Chapter 12 Solutions
Essential Organic Chemistry, Global Edition
Ch. 12.1 - Prob. 1PCh. 12.1 - Name the following compounds:Ch. 12.1 - Prob. 3PCh. 12.1 - Prob. 4PCh. 12.2 - Prob. 5PCh. 12.4 - Draw the structure for each of the following: a....Ch. 12.4 - Prob. 7PCh. 12.5 - What products are formed when the following...Ch. 12.5 - Prob. 9PCh. 12.5 - Prob. 10P
Ch. 12.5 - Prob. 12PCh. 12.5 - Write the mechanism for the reaction of acetyl...Ch. 12.5 - Prob. 14PCh. 12.6 - Prob. 16PCh. 12.7 - Prob. 17PCh. 12.7 - Prob. 18PCh. 12.7 - Prob. 19PCh. 12.8 - Prob. 20PCh. 12.8 - Prob. 21PCh. 12.8 - Prob. 22PCh. 12.9 - Which of the following are a. hemiacetals? b....Ch. 12.9 - Prob. 24PCh. 12.9 - Prob. 25PCh. 12.10 - Prob. 26PCh. 12.11 - Prob. 27PCh. 12 - Draw the structure for each of the following: a....Ch. 12 - Prob. 29PCh. 12 - List the following compounds in order from most...Ch. 12 - Show the reagents required to form the primary...Ch. 12 - Fill in the boxes:Ch. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 34PCh. 12 - Prob. 35PCh. 12 - Prob. 36PCh. 12 - Prob. 37PCh. 12 - Prob. 38PCh. 12 - Prob. 39PCh. 12 - Show two ways the following compound could be...Ch. 12 - Prob. 41PCh. 12 - Prob. 42PCh. 12 - Prob. 43PCh. 12 - Prob. 44PCh. 12 - Prob. 45PCh. 12 - Prob. 46PCh. 12 - Prob. 47PCh. 12 - Prob. 48PCh. 12 - Prob. 49PCh. 12 - Prob. 50PCh. 12 - Prob. 51PCh. 12 - Indicate how the following compounds could be...Ch. 12 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will its major product: 2,0° with a new C-C bond as If this reaction will work, draw the major organic product or products you would expect in the drawing aree below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and desh bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C-C bond, just check the box under the drawing area and leave it blank.arrow_forwardwrite the mechanism of the nucleophilic acyl substitution reaction, please give an examplearrow_forwardThe compound in the figure is reacted with 10 n-butyllihium, 2° propanone, and 3º H2O. Draw and name the products obtained. SiMe3arrow_forward
- Caffeine (C8H10N4O2, pictured below) is a weak base. The pKb of caffeine is 10.4. What is the pH of a 0.0155 M solution of caffeine?arrow_forward2-Cyclopentyl-2-methyl-1,3-dioxolane is reacted with H₂SO₄. Draw and name the structures of the products.arrow_forwardIndicate the products of the reaction of 1-cyclohexyl-2,2-dimethylpropan-1-one with CH3CO3H (). Draw the structures of the compounds.arrow_forward
- Write chemical equations for: the reaction of benzoic acid chloride with grignard reagent [CH3MgX] the reaction of butanoic acid with methyl amine [CH3NH2]arrow_forward2-(3-Aminopropyl)cyclohexan-1-one is reacted with H₂SO₄. Draw the structures of the products.arrow_forwardPlease help me solve number 2arrow_forward
- Choose the best reagents to complete the following reaction. 오 Na2Cr2O7 H2SO4, H2O Problem 22 of 35 A Na2Cr2O7 H2SO4, H2O H2/Pt B pressure OH 1. NaBH4 C 2. H3O+ D DMP (Dess-Martin Periodinane) CH2Cl2 CrO3 Done Dramabana_Minor Submitarrow_forwardIndicate the products of the reaction of Cycloheptanone with pyrrolidine (cat. H+). Draw the structures of the compounds.arrow_forwardIndicate the products of the reaction of 2-(3-aminopropyl)cyclohexan-1-one with H2SO4. Draw the structures of the compounds.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY