
Concept explainers
Draw the structure for each of the following:
- a. isobutyraldehyde
- b. diisopentyl
ketone - c. 3-methylcyclohexanone
- d. 2,4-pentanedione
- e. 4-bromo-3-heptanone
- f. 4-bromohexanal
(a)
Interpretation:
The structure of Isobutyraldehyde has to be drawn.
Concept Introduction:
For any given organic compound name, the corresponding structure can be analysed and drawn by analysing or identifying the following aspects of the name:
- Parent chain.
- Functional group.
- Position of the functional group on the parent chain.
- Alkyl substituent.
- Position of the alkyl substituent on the parent chain.
Answer to Problem 28P
The structure of Isobutyraldehyde is given below:
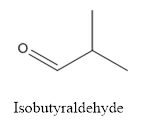
Explanation of Solution
The compound has aldehyde group that is attached to the isobutyl group.
The structure of isobutyraldehyde is as follows.

(b)
Interpretation:
The structure of diisopentyl ketone has to be drawn.
Concept Introduction:
For any given organic compound name, the corresponding structure can be analysed and drawn by analysing or identifying the following aspects of the name:
- Parent chain.
- Functional group.
- Position of the functional group on the parent chain.
- Alkyl substituent.
- Position of the alkyl substituent on the parent chain.
Answer to Problem 28P
The structure of diisopentyl ketone is given below:
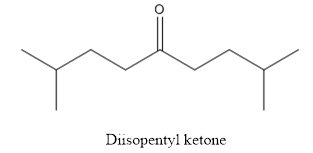
Explanation of Solution
The compound has ketone functional group. Two isopentyl groups are attached to the keto group.
The structure of diisopentyl ketone is as follows.

(c)
Interpretation:
The structure of 3-methylcyclohexanone has to be drawn.
Concept Introduction:
For any given organic compound name, the corresponding structure can be analysed and drawn by analysing or identifying the following aspects of the name:
- Parent chain.
- Functional group.
- Position of the functional group on the parent chain.
- Alkyl substituent.
- Position of the alkyl substituent on the parent chain.
Answer to Problem 28P
The structure of 3-methylcyclohexanoneis given below:
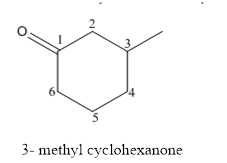
Explanation of Solution
The compound has ketone functional and group and it is cyclohexanone that has one methyl group at 3rd position of the cyclic ring.
The structure of 3-methyl cyclohexanone is as follows.

(d)
Interpretation:
The structure of 2,4-pentanedione has to be drawn.
Concept Introduction:
For any given organic compound name, the corresponding structure can be analysed and drawn by analysing or identifying the following aspects of the name:
- Parent chain.
- Functional group.
- Position of the functional group on the parent chain.
- Alkyl substituent.
- Position of the alkyl substituent on the parent chain.
Answer to Problem 28P
The structure of 2,4-pentanedione is given below:
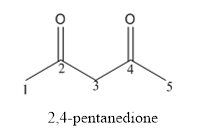
Explanation of Solution
The compound has two keto functional groups and these are attached to the pentane carbon chain.
The structure of 2,4-pentanedione is as follows:

(e)
Interpretation:
The structure of 4-bromo-3-heptanone has to be drawn.
Concept Introduction:
For any given organic compound name, the corresponding structure can be analysed and drawn by analysing or identifying the following aspects of the name:
- Parent chain.
- Functional group.
- Position of the functional group on the parent chain.
- Alkyl substituent.
- Position of the alkyl substituent on the parent chain.
Answer to Problem 28P
The structure of 4-bromo-3-heptanone is given below:
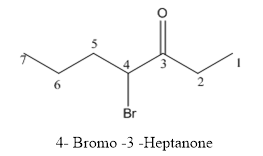
Explanation of Solution
The compound has keto functional group and it is attached to the heptane carbon chain. At the 4th position of the carbon chain has bromine attachment.

(f)
Interpretation:
The structure of 4-bromohexenal has to be drawn.
Concept Introduction:
For any given organic compound name, the corresponding structure can be analysed and drawn by analysing or identifying the following aspects of the name:
- Parent chain.
- Functional group.
- Position of the functional group on the parent chain.
- Alkyl substituent.
- Position of the alkyl substituent on the parent chain.
Answer to Problem 28P
The structure of 4-bromohexenal is given below:
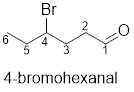
Explanation of Solution
The compound has bromine functional group at the 4th position from the aldehyde functional group on the hexane parent chain.
The structure of 4-bromohexenal is as follows:

Want to see more full solutions like this?
Chapter 12 Solutions
Essential Organic Chemistry, Global Edition
- Step 1: add a curved arrow. Select Draw Templates More / " C H Br 0 Br : :o: Erase H H H H Q2Q Step 2: Draw the intermediates and a curved arrow. Select Draw Templates More MacBook Air / " C H Br 0 9 Q Erase 2Qarrow_forwardO Macmillan Learning Question 23 of 26 > Stacked Step 7: Check your work. Does your synthesis strategy give a substitution reaction with the expected regiochemistry and stereochemistry? Draw the expected product of the forward reaction. - - CN DMF MacBook Air Clearly show stereochemistry. Questionarrow_forwardNH2 1. CH3–MgCl 2. H3O+ ? As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C - C bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new C - C bond. Х ☐: Carrow_forward
- Predict the major products of this organic reaction. If there will be no major products, check the box under the drawing area instead. No reaction. : + Х è OH K Cr O 2 27 2 4' 2 Click and drag to start drawing a structure.arrow_forwardLaminar compounds are characterized by havinga) a high value of the internal surface of the solid.b) a high adsorption potential.arrow_forwardIntercalation compounds have their sheetsa) negatively charged.b) positively charged.arrow_forward
- Indicate whether the following two statements are correct or not:- Polythiazine, formed by N and S, does not conduct electricity- Carbon can have a specific surface area of 3000 m2/garrow_forwardIndicate whether the following two statements are correct or not:- The S8 heterocycle is the origin of a family of compounds- Most of the elements that give rise to stable heterocycles belong to group d.arrow_forwardcould someone draw curly arrow mechanism for this question pleasearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





