
Concept explainers
(a)
Interpretation:
The polar covalent bond in the given molecule is to be identified.
Concept introduction:
Covalent bond is formed by sharing of electrons between the atoms forming the bonds
Polar covalent bond is formed between two different atoms.The bond formed is polar when there is electronegativity difference between the atoms forming the bond due to which atoms gets polarity.More electronegative atom acquires partially negative charge and less electronegative atom acquires partial positive charge.
(a)
Answer to Problem 10A
Explanation of Solution
The structure of water is:
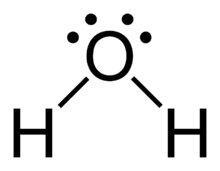
It contains two
(b)
Interpretation:
The polar covalent bond in the given molecule is to be identified.
Concept introduction:
Covalent bond is formed by sharing of electrons between the atoms forming the bonds
Polar covalent bond is formed between two different atoms.The bond formed is polar when there is electronegativity difference between the atoms forming the bond due to which atoms gets polarity.More electronegative atom acquires partially negative charge and less electronegative atom acquires partial positive charge.
(b)
Answer to Problem 10A
Explanation of Solution
The structure of
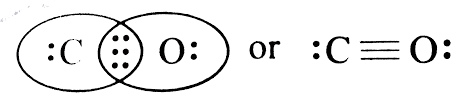
It contains one
(c)
Interpretation:
The polar covalent bond in the given molecule is to be identified.
Concept introduction:
Covalent bond is formed by sharing of electrons between the atoms forming the bonds
Polar covalent bond is formed between two different atoms.The bond formed is polar when there is electronegativity difference between the atoms forming the bond due to which atoms gets polarity.More electronegative atom acquires partially negative charge and less electronegative atom acquires partial positive charge.
(c)
Answer to Problem 10A
Explanation of Solution
The structure of
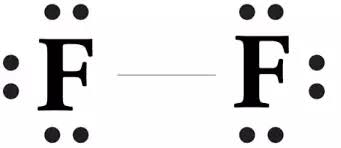
The bond is formed between same atoms. So it is no-polar bond.
(d)
Interpretation:
The polar covalent bond in the given molecule is to be identified.
Concept introduction:
Covalent bond is formed by sharing of electrons between the atoms forming the bonds
Polar covalent bond is formed between two different atoms.The bond formed is polar when there is electronegativity difference between the atoms forming the bond due to which atoms gets polarity.More electronegative atom acquires partially negative charge and less electronegative atom acquires partial positive charge.
(d)
Answer to Problem 10A
Explanation of Solution
The structure of
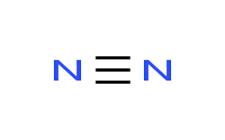
The bond is formed between same atoms. So it is no-polar bond.
Chapter 12 Solutions
World of Chemistry
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





