
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 11.15, Problem 30P
How would you synthesize the following compounds starting with a
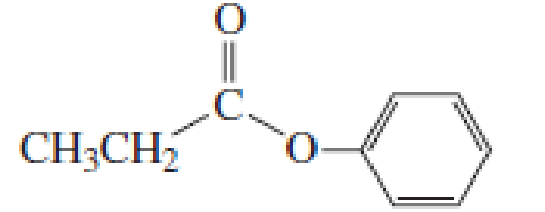
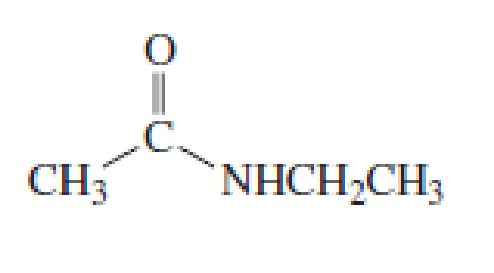
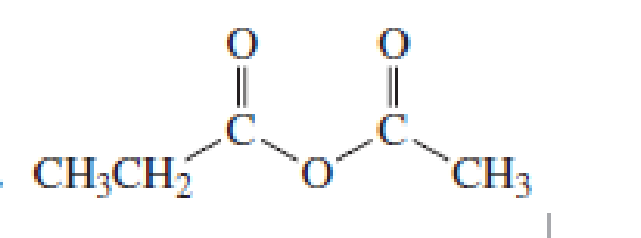
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
#1. Retro-Electrochemical Reaction: A ring has been made, but the light is causing the molecule to un-
cyclize. Undo the ring into all possible molecules. (2pts, no partial credit)
hv
Don't used Ai solution
I have a question about this problem involving mechanisms and drawing curved arrows for acids and bases. I know we need to identify the nucleophile and electrophile, but are there different types of reactions? For instance, what about Grignard reagents and other types that I might not be familiar with? Can you help me with this? I want to identify the names of the mechanisms for problems 1-14, such as Gilman reagents and others. Are they all the same? Also, could you rewrite it so I can better understand? The handwriting is pretty cluttered. Additionally, I need to label the nucleophile and electrophile, but my main concern is whether those reactions differ, like the "Brønsted-Lowry acid-base mechanism, Lewis acid-base mechanism, acid-catalyzed mechanisms, acid-catalyzed reactions, base-catalyzed reactions, nucleophilic substitution mechanisms (SN1 and SN2), elimination reactions (E1 and E2), organometallic mechanisms, and so forth."
Chapter 11 Solutions
Essential Organic Chemistry, Global Edition
Ch. 11.1 - The aromas of many flowers and fruits are due to...Ch. 11.1 - Name the following compounds:Ch. 11.1 - Prob. 3PCh. 11.2 - Prob. 4PCh. 11.2 - Prob. 5PCh. 11.4 - a. What is the product of the reaction of acetyl...Ch. 11.4 - Prob. 7PCh. 11.5 - Using the pKa values listed in Table 11.1, predict...Ch. 11.6 - Starting with acetyl chloride, what neutral...Ch. 11.6 - Prob. 10P
Ch. 11.7 - Prob. 11PCh. 11.8 - Prob. 13PCh. 11.8 - Using the mechanism for the acidcatalyzed...Ch. 11.8 - Prob. 15PCh. 11.8 - Prob. 16PCh. 11.8 - Prob. 17PCh. 11.9 - Prob. 18PCh. 11.10 - Show how each of the following esters could be...Ch. 11.11 - Which of the following reactions would lead to the...Ch. 11.12 - Prob. 22PCh. 11.12 - Prob. 23PCh. 11.13 - Prob. 24PCh. 11.13 - Prob. 25PCh. 11.14 - Prob. 26PCh. 11.14 - Prob. 27PCh. 11.14 - Prob. 28PCh. 11.15 - Prob. 29PCh. 11.15 - How would you synthesize the following compounds...Ch. 11 - Write a structure for each of the following a. N,N...Ch. 11 - Prob. 32PCh. 11 - Which ester is more reactive, methyl acetate or...Ch. 11 - What products would be formed from the reaction of...Ch. 11 - What products would be obtained from the following...Ch. 11 - Prob. 36PCh. 11 - a. Which compound would you expect to have a...Ch. 11 - a. List the following esters in order of...Ch. 11 - D. N. Kursanov, a Russian chemist, proved that the...Ch. 11 - Prob. 40PCh. 11 - Using an alcohol for one method and an alkyl...Ch. 11 - Prob. 42PCh. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Show how the following compounds could be prepared...Ch. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forwardComplete the spectroscopy with structurearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY