
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 11, Problem 37P
- a. Which compound would you expect to have a higher dipole moment, methyl acetate or butanone?
- b. Which would you expect to have a higher boiling point?
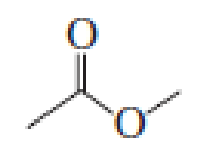
methyl acetate
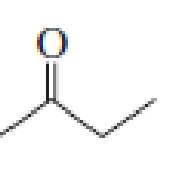
butanone
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. What letter has an aldehyde with molecular formula C₄H₈O.
2. What letter has an ester with molecular formula C₄H₈O₂
3. What letter has a ketone with molecular formula C₄H₈O₂
4. What letter has a carboxylic acid with molecular formula C₄H₈O₂
Instructions: Give the IUPAC name for each compound.
A.
CH₂
CH₂CH₂CH₂CCH₂COOH
CH₂
B.
CH.CHCHCH.COOH
a
CH₂CH₂
C. (CH,CH,), CHCH,CH COOH
Instructions: Give the structure corresponding to each IUPAC name.
a. 2-bromobutanoic acid
b. 2,3-dimethylpentanoic acid
c. 2-ethyl-5,5-dimethyloctanoic acid
d. 3,4,5,6-tetraethyldecanoic acid
For each structure, provide the common name (which happens to be the official IUPAC name). Spelling counts.
The common name of A is:
A.
H3C-
The common name of B is:
В.
но
The common name of C is:
С.
Но-
The common name of D is:
D.
H2N-
B.
Chapter 11 Solutions
Essential Organic Chemistry, Global Edition
Ch. 11.1 - The aromas of many flowers and fruits are due to...Ch. 11.1 - Name the following compounds:Ch. 11.1 - Prob. 3PCh. 11.2 - Prob. 4PCh. 11.2 - Prob. 5PCh. 11.4 - a. What is the product of the reaction of acetyl...Ch. 11.4 - Prob. 7PCh. 11.5 - Using the pKa values listed in Table 11.1, predict...Ch. 11.6 - Starting with acetyl chloride, what neutral...Ch. 11.6 - Prob. 10P
Ch. 11.7 - Prob. 11PCh. 11.8 - Prob. 13PCh. 11.8 - Using the mechanism for the acidcatalyzed...Ch. 11.8 - Prob. 15PCh. 11.8 - Prob. 16PCh. 11.8 - Prob. 17PCh. 11.9 - Prob. 18PCh. 11.10 - Show how each of the following esters could be...Ch. 11.11 - Which of the following reactions would lead to the...Ch. 11.12 - Prob. 22PCh. 11.12 - Prob. 23PCh. 11.13 - Prob. 24PCh. 11.13 - Prob. 25PCh. 11.14 - Prob. 26PCh. 11.14 - Prob. 27PCh. 11.14 - Prob. 28PCh. 11.15 - Prob. 29PCh. 11.15 - How would you synthesize the following compounds...Ch. 11 - Write a structure for each of the following a. N,N...Ch. 11 - Prob. 32PCh. 11 - Which ester is more reactive, methyl acetate or...Ch. 11 - What products would be formed from the reaction of...Ch. 11 - What products would be obtained from the following...Ch. 11 - Prob. 36PCh. 11 - a. Which compound would you expect to have a...Ch. 11 - a. List the following esters in order of...Ch. 11 - D. N. Kursanov, a Russian chemist, proved that the...Ch. 11 - Prob. 40PCh. 11 - Using an alcohol for one method and an alkyl...Ch. 11 - Prob. 42PCh. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Show how the following compounds could be prepared...Ch. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An ether has a lower boiling point than an aldehyde of the same mass (or number of C atoms). Why is this? a. the ether, containing a carbonyl group, interacts more strongly (ether molecule to ether molecule) than does an aldehyde (molecule to molecule), b. The ether, with its single O atom, can H bond to only 2 water molecules. c. The ether, with its single O atom and no attached H atom, does not form H bonds and does not have a dipole for dipole/dipole interaction. d. All of the above.arrow_forward1. Write IUPAC name for each structure below. a. CI b. Br d. نیومده e.arrow_forward1. Which of the following organic compounds is the least polar? a. CH3CH2CHO b. CH3COCH3 c. CH3CH2CH2OH d. CH3CH2OCH3 e. CH3COOH 2. Which reagent can be used to convert acetic anhydride to acetic acid? a. NH3 b. CH3COO- c. CH3OH d. None of these e. H2Oarrow_forward
- 1. Match the following: A. alcohol (phenol) B. Ether CH3-CH2-CHO СН3-СН2-С-СНЗ C. Amine С6Н5-СООН D. aldehyde С2H5-СООСHЗ E. Ketone F. Thiol G. carboxylic acid С6Н5-ОН СНЗ-О-С2Н5 CH3-SH H. Ester C2H5-NH2 I. Amide H-C-NH2 2. Name the following: CH3 CH3-C -CH2- Č -CH3 CH3 CH3 CH3 CH3 CH3-C-CH3 CH3 – C- C=C - C6H5arrow_forward1. Draw two resonance structures for 1,3-cyclopentadiene and naphthalene, respectively. Use appropriate resonance arrows. 1,3-cyclopentadiene Naphthalenearrow_forwardType in the correct IUPAC name and spelling of the following compounds. Please note proper notations (like hypen and commas) to avoid invalidating your answers.arrow_forward
- 1. Determine the conjugate base/acid of each (with solution). What strong base can deprotonate the alcohol: 1-butanol 2-propanol Tert-butyl Alcohol 1-phenolarrow_forwardCH3–C–CH2-CH3 For the compound: a. Circle the carbonyl group in the compound above. b. What is the geometry of the carbon atom of the carbonyl group? What is the hybridization of the carbonyl carbon (sp, sp² or sp³)?. d. Is the compound soluble or insoluble in water? e. Is the compound more or less soluble (in water) than an alcohol? f. Does this compound have a higher or lower bp than an alkane? С.arrow_forwardplease explain well and type if you can...arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY