
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.43QE
For each of the following molecules, complete the Lewis structure and use the VSEPR model to determine the bond angles around each central atom. Note that the drawings are only skeleton structures and may depict the angles incorrectly.
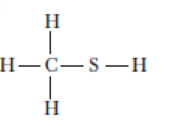
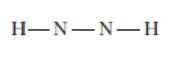
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!
Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!
Can you please explain this problem to me and expand it so I can understand the full Lewis dot structure? Thanks!
Chapter 10 Solutions
Chemistry: Principles and Practice
Ch. 10 - Prob. 10.1QECh. 10 - Prob. 10.2QECh. 10 - Prob. 10.3QECh. 10 - Prob. 10.4QECh. 10 - Prob. 10.5QECh. 10 - Prob. 10.6QECh. 10 - Prob. 10.7QECh. 10 - Prob. 10.8QECh. 10 - Prob. 10.9QECh. 10 - Prob. 10.10QE
Ch. 10 - Which atomic orbitals overlap to form the bonds in...Ch. 10 - Prob. 10.12QECh. 10 - Identify the hybrid orbitals used by boron in BCl3...Ch. 10 - Identify the hybrid orbitals used by antimony in...Ch. 10 - Prob. 10.15QECh. 10 - Prob. 10.16QECh. 10 - Prob. 10.17QECh. 10 - Prob. 10.18QECh. 10 - Prob. 10.19QECh. 10 - Prob. 10.20QECh. 10 - Compare and contrast the molecular orbital and...Ch. 10 - Describe the bonding in molecular orbital terms...Ch. 10 - Prob. 10.23QECh. 10 - Prob. 10.24QECh. 10 - Prob. 10.25QECh. 10 - Prob. 10.26QECh. 10 - Prob. 10.27QECh. 10 - Prob. 10.28QECh. 10 - Prob. 10.29QECh. 10 - Prob. 10.30QECh. 10 - Prob. 10.31QECh. 10 - Prob. 10.32QECh. 10 - Prob. 10.33QECh. 10 - Prob. 10.34QECh. 10 - Prob. 10.35QECh. 10 - Prob. 10.36QECh. 10 - Prob. 10.37QECh. 10 - Prob. 10.38QECh. 10 - Prob. 10.39QECh. 10 - Use the VSEPR model to predict the bond angles...Ch. 10 - Prob. 10.41QECh. 10 - Prob. 10.42QECh. 10 - For each of the following molecules, complete the...Ch. 10 - Prob. 10.44QECh. 10 - Prob. 10.45QECh. 10 - Prob. 10.46QECh. 10 - Indicate which molecules are polar and which are...Ch. 10 - Prob. 10.48QECh. 10 - Indicate which of the following molecules are...Ch. 10 - Prob. 10.50QECh. 10 - Prob. 10.51QECh. 10 - Prob. 10.52QECh. 10 - Prob. 10.53QECh. 10 - Prob. 10.54QECh. 10 - Prob. 10.55QECh. 10 - Prob. 10.56QECh. 10 - Prob. 10.57QECh. 10 - Prob. 10.58QECh. 10 - Prob. 10.59QECh. 10 - Prob. 10.60QECh. 10 - Prob. 10.61QECh. 10 - Prob. 10.62QECh. 10 - Prob. 10.63QECh. 10 - Prob. 10.64QECh. 10 - Prob. 10.65QECh. 10 - Prob. 10.66QECh. 10 - Prob. 10.67QECh. 10 - Prob. 10.68QECh. 10 - Prob. 10.69QECh. 10 - Prob. 10.70QECh. 10 - Prob. 10.71QECh. 10 - Prob. 10.72QECh. 10 - Identify the orbitals on each of the atoms that...Ch. 10 - Prob. 10.74QECh. 10 - Prob. 10.75QECh. 10 - How many sigma bonds and how many pi bonds are...Ch. 10 - Give the hybridization of each central atom in the...Ch. 10 - Prob. 10.78QECh. 10 - Prob. 10.79QECh. 10 - Prob. 10.80QECh. 10 - Prob. 10.81QECh. 10 - Predict the hybridization at each central atom in...Ch. 10 - Prob. 10.83QECh. 10 - Tetrafluoroethylene, C2F4, is used to produce...Ch. 10 - Prob. 10.85QECh. 10 - Prob. 10.86QECh. 10 - Prob. 10.87QECh. 10 - Prob. 10.88QECh. 10 - Prob. 10.89QECh. 10 - Prob. 10.90QECh. 10 - Prob. 10.91QECh. 10 - Prob. 10.92QECh. 10 - Prob. 10.93QECh. 10 - Prob. 10.94QECh. 10 - Prob. 10.95QECh. 10 - Prob. 10.96QECh. 10 - Prob. 10.97QECh. 10 - Prob. 10.98QECh. 10 - The molecular orbital diagram of NO shown in...Ch. 10 - The molecular orbital diagram of NO shown in...Ch. 10 - The molecular orbital diagram of NO shown in...Ch. 10 - Prob. 10.102QECh. 10 - Prob. 10.103QECh. 10 - Prob. 10.104QECh. 10 - Prob. 10.105QECh. 10 - Following are the structures of three isomers of...Ch. 10 - The ions ClF2 and ClF2+ have both been observed....Ch. 10 - Aspirin, or acetylsalicylic acid, has the formula...Ch. 10 - Aspartame is a compound that is 200 times sweeter...Ch. 10 - Prob. 10.110QECh. 10 - Prob. 10.111QECh. 10 - Calcium cyanamide, CaNCN, is used both to kill...Ch. 10 - Histidine is an essential amino acid that the body...Ch. 10 - Formamide, HC(O)NH2, is prepared at high pressures...Ch. 10 - Prob. 10.115QECh. 10 - Prob. 10.116QECh. 10 - Prob. 10.117QECh. 10 - Prob. 10.118QECh. 10 - Prob. 10.119QECh. 10 - Prob. 10.120QECh. 10 - Prob. 10.121QECh. 10 - Prob. 10.122QECh. 10 - Prob. 10.123QECh. 10 - Prob. 10.124QECh. 10 - Two compounds have the formula S2F2. Disulfur...Ch. 10 - Prob. 10.126QECh. 10 - Prob. 10.127QE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer the questions in the photos and please revise any wrong answers. Thank youarrow_forward(Please be sure that 7 carbons are available in the structure )Based on the 1H NMR, 13C NMR, DEPT 135 NMR and DEPT 90 NMR, provide a reasoning step and arrive at the final structure of an unknown organic compound containing 7 carbons. Dept 135 shows peak to be positive at 128.62 and 13.63 Dept 135 shows peak to be negative at 130.28, 64.32, 30.62 and 19.10.arrow_forward-lease help me answer the questions in the photo.arrow_forward
- For the reaction below, the concentrations at equilibrium are [SO₂] = 0.50 M, [0] = 0.45 M, and [SO3] = 1.7 M. What is the value of the equilibrium constant, K? 2SO2(g) + O2(g) 2SO3(g) Report your answer using two significant figures. Provide your answer below:arrow_forwardI need help with this question. Step by step solution, please!arrow_forwardZn(OH)2(s) Zn(OH)+ Ksp = 3 X 10-16 B₁ = 1 x 104 Zn(OH)2(aq) B₂ = 2 x 1010 Zn(OH)3 ẞ3-8 x 1013 Zn(OH) B4-3 x 1015arrow_forward
- Help me understand this by showing step by step solution.arrow_forwardscratch paper, and the integrated rate table provided in class. our scratch work for this test. Content attribution 3/40 FEEDBACK QUESTION 3 - 4 POINTS Complete the equation that relates the rate of consumption of H+ and the rate of formation of Br2 for the given reaction. 5Br (aq) + BrO3 (aq) + 6H (aq) →3Br2(aq) + 3H2O(l) • Your answers should be whole numbers or fractions without any decimal places. Provide your answer below: Search 尚 5 fn 40 * 00 99+ 2 9 144 a [arrow_forward(a) Write down the structure of EDTA molecule and show the complex structure with Pb2+ . (b) When do you need to perform back titration? (c) Ni2+ can be analyzed by a back titration using standard Zn2+ at pH 5.5 with xylenol orange indicator. A solution containing 25.00 mL of Ni2+ in dilute HCl is treated with 25.00 mL of 0.05283 M Na2EDTA. The solution is neutralized with NaOH, and the pH is adjusted to 5.5 with acetate buffer. The solution turns yellow when a few drops of indicator are added. Titration with 0.02299 M Zn2+ requires 17.61 mL to reach the red end point. What is the molarity of Ni2+ in the unknown?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY