
Concept explainers
Interpretation:
Lewis structure for ethylene oxide, ethylene glycol, and acrylonitrile has to be drawn and also the hybrid orbitals of the central atom have to be given along with the number of pi bonds present.
Concept Introduction:
Lewis structure is used for predicting the shape of molecules. From the steric number obtained in a Lewis structure, the molecular geometry can be predicted. VSEPR model can predict the shape of molecules considering their Lewis structure. Certain rules has to be followed in for the VSEPR model.
- The molecule will have a shape where there is minimal electrostatic repulsion between the valence‑shell electron pairs.
- The forces of repulsion between two lone pairs of electrons will be higher than the repulsion between lone pair and bond pair of electrons. This in turn will be higher than the bond pair‑bond pair of electrons.
The hybridized orbitals and the steric number can be related as shown below;
| Steric number | Hybridized orbital |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 |
Explanation of Solution
Ethylene oxide:
Formula for ethylene oxide is
The total number of valence electrons is calculated as shown below;
A total of
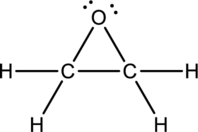
Hybrid orbitals of central carbon atoms:
The carbon atom has does not have a lone pair of electrons and it is bonded to four atoms. Therefore, the steric number is calculated as shown below;
As the steric number is four, the hybridization of carbon atom is
Hybrid orbitals of central oxygen atom:
The oxygen atom has two lone pair of electrons and it is bonded to two atoms. Therefore, the steric number is calculated as shown below;
As the steric number is four, the hybridization of oxygen atom is
There is no unhybridized orbital left out for the formation of pi bonds. Hence, there is no pi bonds in ethylene oxide.
Ethylene glycol:
Formula for ethylene glycol is
The total number of valence electrons is calculated as shown below;
A total of
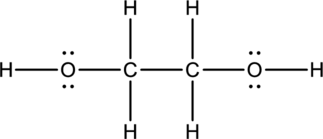
Hybrid orbitals of central carbon atoms:
The carbon atom has does not have a lone pair of electrons and it is bonded to four atoms. Therefore, the steric number is calculated as shown below;
As the steric number is four, the hybridization of carbon atom is
Hybrid orbitals of central oxygen atoms:
The oxygen atom has two lone pair of electrons and it is bonded to two atoms. Therefore, the steric number is calculated as shown below;
As the steric number is four, the hybridization of oxygen atom is
There is no unhybridized orbital left out for the formation of pi bonds. Hence, there is no pi bonds in ethylene glycol.
Acrylonitrile:
Formula for acrylonitrile is
The total number of valence electrons is calculated as shown below;
A total of
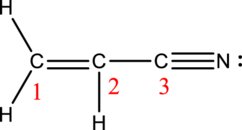
Hybrid orbitals of carbon atom C-1:
The carbon atom has does not have a lone pair of electrons and it is bonded to three atoms. Therefore, the steric number is calculated as shown below;
As the steric number is three, the hybridization of carbon atom is
Hybrid orbitals of carbon atom C-2:
The carbon atom has does not have a lone pair of electrons and it is bonded to three atoms. Therefore, the steric number is calculated as shown below;
As the steric number is three, the hybridization of carbon atom is
Hybrid orbitals of carbon atom C-3:
The carbon atom has does not have a lone pair of electrons and it is bonded to two atoms. Therefore, the steric number is calculated as shown below;
As the steric number is two, the hybridization of carbon atom is
There are one double bond and one triple bond present in the structure. As multiple bonds are present, acrylonitrile contains pi bonds.
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry: Principles and Practice
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





