
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.63P
Draw in all the carbon and hydrogen atoms in each molecule.
a. 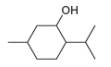 c.
c. 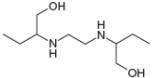
methanol ethambutol
(isolated from peppermint oil) (drug used to treat tuberculosis)
b. 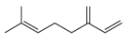 d.
d. 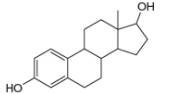
myrcene estradiol
(isolated from bayberry) (a female sex harmone)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Using dashed line wedge projections drawthe indicated compounds and indicate whether thecompound you have drawn is R or S.(a) The two enantiomers of 2-chlorobutane. Can you please explain your steps and how you would approach a similar problem. Thank you!
5)
There are no lone pairs shown in the structure below. Please add in all lone pairs and then give the
hybridization scheme for the compound. (8)
10,11
7)
1.2.3
H
4
| 14
8)
COC
12
13
H
16
15
H7
9)
-
5.6
C
8
H
10)
H
1).
2)
3)_
11)
12)
13)
4)_
14)
5)
15)
16)
6)
The sum of the numbers in the name of isA. 11; B. 13; C. 10; D. 12; E. none of the other answers iscorrect. I believe the awnser should be E to this problem but the solution to this problem is D 12. I'm honestly unsure how that's the solution. If you can please explain the steps to this type of problem and how to approach a problem like this it would be greatly appreciated!
Chapter 1 Solutions
Organic Chemistry-Package(Custom)
Ch. 1 - While the most common isotope of nitrogen has a...Ch. 1 - Label each bond in the following compounds as...Ch. 1 - How many covalent bonds are predicted for each...Ch. 1 - Draw a valid Lewis structure for each species. a....Ch. 1 - Draw an acceptable Lewis structure for each...Ch. 1 - Prob. 1.6PCh. 1 - Draw a Lewis structure for each ion. a. CH3Ob....Ch. 1 - Draw Lewis structures for each molecular formula....Ch. 1 - Prob. 1.9PCh. 1 - Prob. 1.10P
Ch. 1 - Prob. 1.11PCh. 1 - Prob. 1.12PCh. 1 - Draw a second resonance structure for each...Ch. 1 - Prob. 1.14PCh. 1 - Draw a second resonance structure for nitrous...Ch. 1 - Prob. 1.16PCh. 1 - Prob. 1.17PCh. 1 - Prob. 1.18PCh. 1 - Prob. 1.19PCh. 1 - Prob. 1.20PCh. 1 - Simplify each condensed structure by using...Ch. 1 - Prob. 1.22PCh. 1 - Prob. 1.23PCh. 1 - Convert each skeletal structure to a complete...Ch. 1 - Draw in all hydrogens and lone pairs on the...Ch. 1 - Prob. 1.26PCh. 1 - What orbitals are used to form each of the CC, and...Ch. 1 - What orbitals are used to form each bond in the...Ch. 1 - Determine the hybridization around the highlighted...Ch. 1 - Classify each bond in the following molecules as ...Ch. 1 - Prob. 1.31PCh. 1 - Rank the following atoms in order of increasing...Ch. 1 - Prob. 1.33PCh. 1 - Prob. 1.34PCh. 1 - Provide the following information about...Ch. 1 - Use the ball-and-stick model to answer each...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Two radioactive isotopes of iodine used for the...Ch. 1 - Prob. 1.40PCh. 1 - Assign formal charges to each carbon atom in the...Ch. 1 - Assign formal charges to each N and O atom in the...Ch. 1 - Draw one valid Lewis structure for each compound....Ch. 1 - Prob. 1.44PCh. 1 - Prob. 1.45PCh. 1 - Prob. 1.46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 1.49PCh. 1 - Prob. 1.50PCh. 1 - Prob. 1.51PCh. 1 - Prob. 1.52PCh. 1 - Prob. 1.53PCh. 1 - Prob. 1.54PCh. 1 - Draw all reasonable resonance structures for each...Ch. 1 - Prob. 1.56PCh. 1 - Rank the resonance structures in each group in...Ch. 1 - 1.56 Consider the compounds and ions with curved...Ch. 1 - 1.57 Predict all bond angles in each...Ch. 1 - Predict the geometry around each indicated atom....Ch. 1 - Prob. 1.61PCh. 1 - Prob. 1.62PCh. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - Prob. 1.64PCh. 1 - Prob. 1.65PCh. 1 - Prob. 1.66PCh. 1 - Prob. 1.67PCh. 1 - Each of the following condensed or skeletal...Ch. 1 - Prob. 1.69PCh. 1 - Prob. 1.70PCh. 1 - Prob. 1.71PCh. 1 - Prob. 1.72PCh. 1 - Prob. 1.73PCh. 1 - Prob. 1.74PCh. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Label the polar bonds in each molecule. Indicate...Ch. 1 - Answer the following questions about acetonitrile...Ch. 1 - Prob. 1.79PCh. 1 - The principles of this chapter can be applied to...Ch. 1 -
a. What is the hybridization of each N atom in...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 1.85PCh. 1 - Prob. 1.86PCh. 1 - Prob. 1.87PCh. 1 - Prob. 1.88PCh. 1 - Prob. 1.89PCh. 1 - Prob. 1.90P
Additional Science Textbook Solutions
Find more solutions based on key concepts
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
What process causes the Mediterranean intermediate Water MIW to become more dense than water in the adjacent At...
Applications and Investigations in Earth Science (9th Edition)
Why are mutants used as test organisms in the Ames test?
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following data for phosphorus: g atomic mass 30.974 mol electronegativity 2.19 kJ electron affinity 72. mol kJ ionization energy 1011.8 mol kJ heat of fusion 0.64 mol You may find additional useful data in the ALEKS Data tab. Does the following reaction absorb or release energy? 2+ + (1) P (g) + e → P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (1) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (1): Does the following reaction absorb or release energy? 00 release absorb Can't be decided with the data given. yes no ☐ kJ/mol (²) P* (8) + + + e →>> P (g) Is it possible to calculate the amount of energy absorbed or released by reaction (2) using only the data above? If you answered yes to the previous question, enter the amount of energy absorbed or released by reaction (2): ☐ release absorb Can't be decided with the data given. yes no kJ/mol аarrow_forwardThe number of hydrogens in an alkyne that has a main chain of 14carbons to which are attached a cyclobutyl ring, a benzene ring, an–OH group, and a Br is A. 34; B. 35; C. 36; D. 24; E. 43arrow_forwardHello! I have a 500 Hz H-NMR for 1,5-bis-(4-methoxyphenyl)-penta-1,4-dien-3-one. I need to label the signals with the corresponding H's. Then, find out if the two alkenes are cis or trans by calculating the J values. I believe that I have the H-NMR labeled correctly, but not sure if I got the J values correct to determine if the two alkenes in the compound will make the compound cis or trans.arrow_forward
- 7) Please use MO diagrams in your explanations. (10) a) If you remove one electron from O₂, b) does this weaken or strengthen the bond? What charge would N, need to have in order for its Bond Order to be 2.5?arrow_forwardpls helparrow_forwardDon't used hand raiting and don't used Ai solution and correct answerarrow_forward
- Don't used hand raiting and don't used Ai solution and correct answerarrow_forwardPredict the product formed when the compound shown below undergoes a reaction with MCPBA in CH2Cl2. MCPBA is meta-chloroperoxybenzoic acid.arrow_forwardk https://app.aktiv.com STARTING AMOUNT 6 58°F Clear + F1 X Dimensional Analysis - Aktiv Chemistry Your Aktiv Learning trial expires on 02/25/25 at 02:14 PM Question 19 of 22 Polyethylene terephthalate (PET) is used in plastic water bottles. A water bottle has a mass of 14.0 grams. Given a density of 1.38 g/cm³, what is the volume of the plastic used to make the water bottle in cm³ ? ADD FACTOR ANSWER RESET ว 100 14.0 0.01 10.1 1000 0.099 1.38 0.001 Q Search F5 -O+ F6 F7 + F3 F2 W E S4 ST #3 F4 % 5 Y R S & 7 cm³ g/cm³ g ם F8 * 00 8 F9 P ل DOD S F10 F11 F12 Insert D F G H J K + 11arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY