
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.68P
Each of the following condensed or skeletal structures is an incorrect representation of a molecule or ion. Explain what is wrong in each structure.
a. CH3CH=CH=CHCH3
b. 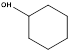 (CH3)3CHCH2CH2CH3
(CH3)3CHCH2CH2CH3
c.

d.
![]()
e
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can someone help me understand this?
help w d!
Help w b!
Chapter 1 Solutions
Organic Chemistry-Package(Custom)
Ch. 1 - While the most common isotope of nitrogen has a...Ch. 1 - Label each bond in the following compounds as...Ch. 1 - How many covalent bonds are predicted for each...Ch. 1 - Draw a valid Lewis structure for each species. a....Ch. 1 - Draw an acceptable Lewis structure for each...Ch. 1 - Prob. 1.6PCh. 1 - Draw a Lewis structure for each ion. a. CH3Ob....Ch. 1 - Draw Lewis structures for each molecular formula....Ch. 1 - Prob. 1.9PCh. 1 - Prob. 1.10P
Ch. 1 - Prob. 1.11PCh. 1 - Prob. 1.12PCh. 1 - Draw a second resonance structure for each...Ch. 1 - Prob. 1.14PCh. 1 - Draw a second resonance structure for nitrous...Ch. 1 - Prob. 1.16PCh. 1 - Prob. 1.17PCh. 1 - Prob. 1.18PCh. 1 - Prob. 1.19PCh. 1 - Prob. 1.20PCh. 1 - Simplify each condensed structure by using...Ch. 1 - Prob. 1.22PCh. 1 - Prob. 1.23PCh. 1 - Convert each skeletal structure to a complete...Ch. 1 - Draw in all hydrogens and lone pairs on the...Ch. 1 - Prob. 1.26PCh. 1 - What orbitals are used to form each of the CC, and...Ch. 1 - What orbitals are used to form each bond in the...Ch. 1 - Determine the hybridization around the highlighted...Ch. 1 - Classify each bond in the following molecules as ...Ch. 1 - Prob. 1.31PCh. 1 - Rank the following atoms in order of increasing...Ch. 1 - Prob. 1.33PCh. 1 - Prob. 1.34PCh. 1 - Provide the following information about...Ch. 1 - Use the ball-and-stick model to answer each...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Two radioactive isotopes of iodine used for the...Ch. 1 - Prob. 1.40PCh. 1 - Assign formal charges to each carbon atom in the...Ch. 1 - Assign formal charges to each N and O atom in the...Ch. 1 - Draw one valid Lewis structure for each compound....Ch. 1 - Prob. 1.44PCh. 1 - Prob. 1.45PCh. 1 - Prob. 1.46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 1.49PCh. 1 - Prob. 1.50PCh. 1 - Prob. 1.51PCh. 1 - Prob. 1.52PCh. 1 - Prob. 1.53PCh. 1 - Prob. 1.54PCh. 1 - Draw all reasonable resonance structures for each...Ch. 1 - Prob. 1.56PCh. 1 - Rank the resonance structures in each group in...Ch. 1 - 1.56 Consider the compounds and ions with curved...Ch. 1 - 1.57 Predict all bond angles in each...Ch. 1 - Predict the geometry around each indicated atom....Ch. 1 - Prob. 1.61PCh. 1 - Prob. 1.62PCh. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - Prob. 1.64PCh. 1 - Prob. 1.65PCh. 1 - Prob. 1.66PCh. 1 - Prob. 1.67PCh. 1 - Each of the following condensed or skeletal...Ch. 1 - Prob. 1.69PCh. 1 - Prob. 1.70PCh. 1 - Prob. 1.71PCh. 1 - Prob. 1.72PCh. 1 - Prob. 1.73PCh. 1 - Prob. 1.74PCh. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Label the polar bonds in each molecule. Indicate...Ch. 1 - Answer the following questions about acetonitrile...Ch. 1 - Prob. 1.79PCh. 1 - The principles of this chapter can be applied to...Ch. 1 -
a. What is the hybridization of each N atom in...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 1.85PCh. 1 - Prob. 1.86PCh. 1 - Prob. 1.87PCh. 1 - Prob. 1.88PCh. 1 - Prob. 1.89PCh. 1 - Prob. 1.90P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- b. CH3 H3C 'N' H3C CH3 CN Ph 1. OH N 2. H2O2, Pyridinearrow_forwardFor each of the Followin, moleaks draw all OF The Resonance contributing stuluctures and compare these three molecules in terms of Resonance stabilization 1-C-1 a. b. H A-C+ О 112-1 C. F-C-F Farrow_forwarda. Explain Why electron withdrawing groupe tend to be meta-Directors. Your answer Should lyclude all apropriate. Resonance contributing Structures 6. Explain why -ll is an ortho -pura drccton evon though chlorine has a very High Electronegativityarrow_forward
- Question 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forwardElectrochemistry. Briefly describe the Donnan potential.arrow_forwardIndicate what the Luther equation is used for?arrow_forward
- Indicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.arrow_forwardAt an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY