
Concept explainers
Zingerone gives ginger its pungent taste.
a. What is the molecular formula of zingerone?
b. How many lone pairs are present?
c. Draw a skeletal structure.
d. How many
e. What orbitals are used to form each indicated bond
(a)
Interpretation: The molecular formula of zingerone is to be stated.
Concept introduction: In ball-and-stick model, each colored ball represents a specific atom and each stick represents a bond. In this model, each black ball represents
Answer to Problem 1.38P
The molecular formula of zingerone is
Explanation of Solution
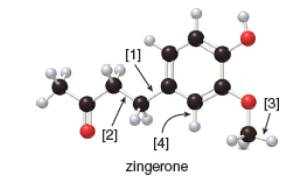
The given ball-and-stick model of zingerone is,
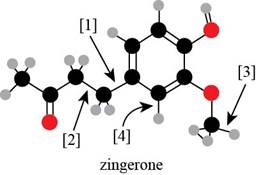
Figure 1
In ball-and-stick model, each colored ball represents a specific atom and each stick represents a bond. In this model, each black ball represents
In the above model,
• There are three red balls. Thus, there are three
• There are eleven black balls. Thus, there are eleven
• There are fourteen grey balls. Thus, there are fourteen
Hence, the molecular formula of zingerone is
The molecular formula of zingerone is
(b)
Interpretation: The number of lone pairs in zingerone is to be stated.
Concept introduction: In a compound or molecule, the lone pairs represent number of unshared electrons on atom. An atom may or may not have unshared electrons. For example, carbon and hydrogen atoms have no lone pair but each oxygen atom has two lone pairs.
Answer to Problem 1.38P
There are total
Explanation of Solution
The molecular formula of citric acid is
There are total
(c)
Interpretation: A skeletal structure of zingerone is to be drawn.
Concept introduction: A ball-and-stick model is converted into skeletal structure by replacing black ball with
Answer to Problem 1.38P
A skeletal structure of zingerone is,
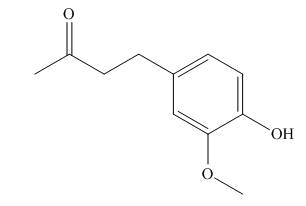
Explanation of Solution
In ball-and-stick model each colored ball represents a specific atom and each stick represents a bond. A ball-and-stick model is converted into skeletal structure by replacing black ball with
A skeletal structure of zingerone is shown in Figure 2.

Figure 2
In ball-and-stick model each colored ball represents a specific atom and each stick represents a bond.
(d)
Interpretation: The number of
Concept introduction: According to the rule of hybridization, an atom that is surrounded with two groups is
Answer to Problem 1.38P
There are seven
Explanation of Solution
The Lewis structure of zingerone is,
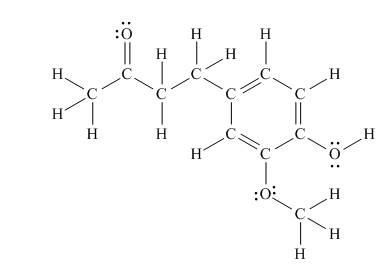
Figure 3
According to the rules of hybridization, an atom that is surrounded with two groups is
The
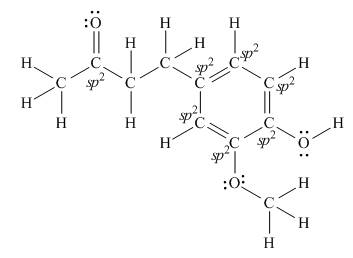
Figure 4
Thus, there are seven
There are seven
(e)
Interpretation: The orbitals that are used to form each indicated bond is to be stated.
Concept introduction: According to the rule of hybridization, an atom that is surrounded with two groups is
Answer to Problem 1.38P
Bond
Explanation of Solution
Bond [1] represents bonding between the carbon atom of benzene
Thus,
Bond
Bond
Thus,
Bond
Thus, bond
The number of surrounded group around any atom predicts the hybridization of that atom, which is further helpful in predicting the orbitals involved in the bond formation.
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry-Package(Custom)
Additional Science Textbook Solutions
Campbell Essential Biology with Physiology (5th Edition)
General, Organic, and Biological Chemistry - 4th edition
HUMAN ANATOMY
Genetic Analysis: An Integrated Approach (3rd Edition)
Microbiology: An Introduction
- QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forwarder your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward
- 5.arrow_forward6.arrow_forward0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward
- 5.arrow_forward9arrow_forwardalekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward
- 0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forwardx + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forwardS: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward

 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning




