
Concept explainers
Draw one valid Lewis structure for each compound. Assume the atoms are arranged as drawn.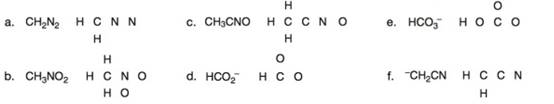
(a)
Interpretation: A valid Lewis structure for the given compound is to be drawn.
Concept introduction: The arrangement of valence electrons among the atoms present in a molecule is represented by the Lewis structure of that molecule. The electron pairs in the molecule are considered to be delocalized.
Answer to Problem 1.43P
The valid Lewis structure for the given compound is,
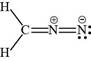
Explanation of Solution
The given compound is
![]()
Figure 1
The valence electrons are counted as,
The bonds and lone pairs are added in the given arrangement of atoms. The valid Lewis structure is,

Figure 2
The valid Lewis structure for the given compound is shown in Figure 2.
(b)
Interpretation: A valid Lewis structure for the given compound is to be drawn.
Concept introduction: The arrangement of valence electrons among the atoms present in a molecule is represented by the Lewis structure of that molecule. The electron pairs in the molecule are considered to be delocalized.
Answer to Problem 1.43P
The valid Lewis structure for the given compound is,
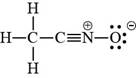
Explanation of Solution
The given compound is
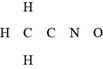
Figure 3
The valence electrons are counted as,
The bonds and lone pairs are added in the given arrangement of atoms. The valid Lewis structure is,

Figure 4
The valid Lewis structure for the given compound is shown in Figure 4.
(c)
Interpretation: A valid Lewis structure for the given compound is to be drawn.
Concept introduction: The arrangement of valence electrons among the atoms present in a molecule is represented by the Lewis structure of that molecule. The electron pairs in the molecule are considered to be delocalized.
Answer to Problem 1.43P
The valid Lewis structure for the given compound is,
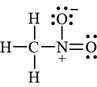
Explanation of Solution
The given compound is
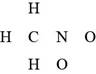
Figure 5
The valence electrons are counted as,
The bonds and lone pairs are added in the given arrangement of atoms. The valid Lewis structure is,
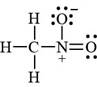
Figure 6
The valid Lewis structure for the given compound is shown in Figure 6.
(d)
Interpretation: A valid Lewis structure for the given compound is to be drawn.
Concept introduction: The arrangement of valence electrons among the atoms present in a molecule is represented by the Lewis structure of that molecule. The electron pairs in the molecule are considered to be delocalized.
Answer to Problem 1.43P
The valid Lewis structure for the given compound is,
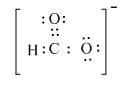
Explanation of Solution
The given compound is
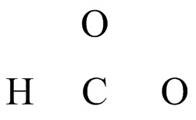
Figure 7
The valence electrons are counted as,
The bonds and lone pairs are added in the given arrangement of atoms. The valid Lewis structure is,
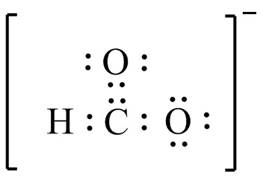
Figure 8
The valid Lewis structure for the given compound is shown in Figure 8.
(e)
Interpretation: A valid Lewis structure for the given compound is to be drawn.
Concept introduction: The arrangement of valence electrons among the atoms present in a molecule is represented by the Lewis structure of that molecule. The electron pairs in the molecule are considered to be delocalized.
Answer to Problem 1.43P
The valid Lewis structure for the given compound is,
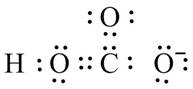
Explanation of Solution
The given compound is
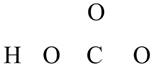
Figure 9
The valence electrons are counted as,
The bonds and lone pairs are added in the given arrangement of atoms. The valid Lewis structure is,
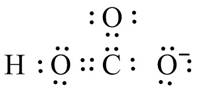
Figure 10
The valid Lewis structure for the given compound is shown in Figure 10.
(f)
Interpretation: A valid Lewis structure for the given compound is to be drawn.
Concept introduction: The arrangement of valence electrons among the atoms present in a molecule is represented by the Lewis structure of that molecule. The electron pairs in the molecule are considered to be delocalized.
Answer to Problem 1.43P
The valid Lewis structure for the given compound is,
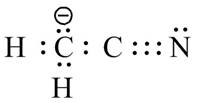
Explanation of Solution
The given compound is
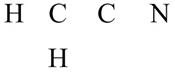
Figure 11
The valence electrons are counted as,
The bonds and lone pairs are added in the given arrangement of atoms. The valid Lewis structure is,
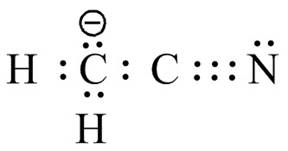
Figure 12
The valid Lewis structure for the given compound is shown in Figure 12.
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry-Package(Custom)
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





