
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.24P
Convert each skeletal structure to a complete structure with all
a. 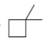 b.
b. 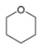 c.
c. 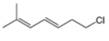 d.
d. 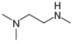
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What are the monomers used to make the following polymers?
F.
а.
b.
с.
d.
Вецер
хочому
な
1. Propose a reasonable mechanism for the following transformation. I'm looking for curved
mechanistic arrows and appropriate formal charges on intermediates.
OMe
MeO OMe
Me2N
NMe2
OTBS
OH
xylenes
OMe
'OTBS
What is the polymer made from the following monomers? What type of polymerization is
used for each?
а.
ОН
H2N
но
b.
ن
-NH2
d.
H₂N
NH2
дов
Chapter 1 Solutions
Organic Chemistry-Package(Custom)
Ch. 1 - While the most common isotope of nitrogen has a...Ch. 1 - Label each bond in the following compounds as...Ch. 1 - How many covalent bonds are predicted for each...Ch. 1 - Draw a valid Lewis structure for each species. a....Ch. 1 - Draw an acceptable Lewis structure for each...Ch. 1 - Prob. 1.6PCh. 1 - Draw a Lewis structure for each ion. a. CH3Ob....Ch. 1 - Draw Lewis structures for each molecular formula....Ch. 1 - Prob. 1.9PCh. 1 - Prob. 1.10P
Ch. 1 - Prob. 1.11PCh. 1 - Prob. 1.12PCh. 1 - Draw a second resonance structure for each...Ch. 1 - Prob. 1.14PCh. 1 - Draw a second resonance structure for nitrous...Ch. 1 - Prob. 1.16PCh. 1 - Prob. 1.17PCh. 1 - Prob. 1.18PCh. 1 - Prob. 1.19PCh. 1 - Prob. 1.20PCh. 1 - Simplify each condensed structure by using...Ch. 1 - Prob. 1.22PCh. 1 - Prob. 1.23PCh. 1 - Convert each skeletal structure to a complete...Ch. 1 - Draw in all hydrogens and lone pairs on the...Ch. 1 - Prob. 1.26PCh. 1 - What orbitals are used to form each of the CC, and...Ch. 1 - What orbitals are used to form each bond in the...Ch. 1 - Determine the hybridization around the highlighted...Ch. 1 - Classify each bond in the following molecules as ...Ch. 1 - Prob. 1.31PCh. 1 - Rank the following atoms in order of increasing...Ch. 1 - Prob. 1.33PCh. 1 - Prob. 1.34PCh. 1 - Provide the following information about...Ch. 1 - Use the ball-and-stick model to answer each...Ch. 1 - Citric acid is responsible for the tartness of...Ch. 1 - Zingerone gives ginger its pungent taste. a.What...Ch. 1 - Two radioactive isotopes of iodine used for the...Ch. 1 - Prob. 1.40PCh. 1 - Assign formal charges to each carbon atom in the...Ch. 1 - Assign formal charges to each N and O atom in the...Ch. 1 - Draw one valid Lewis structure for each compound....Ch. 1 - Prob. 1.44PCh. 1 - Prob. 1.45PCh. 1 - Prob. 1.46PCh. 1 - Draw all possible isomers for each molecular...Ch. 1 - 1.45 Draw Lewis structures for the nine isomers...Ch. 1 - Prob. 1.49PCh. 1 - Prob. 1.50PCh. 1 - Prob. 1.51PCh. 1 - Prob. 1.52PCh. 1 - Prob. 1.53PCh. 1 - Prob. 1.54PCh. 1 - Draw all reasonable resonance structures for each...Ch. 1 - Prob. 1.56PCh. 1 - Rank the resonance structures in each group in...Ch. 1 - 1.56 Consider the compounds and ions with curved...Ch. 1 - 1.57 Predict all bond angles in each...Ch. 1 - Predict the geometry around each indicated atom....Ch. 1 - Prob. 1.61PCh. 1 - Prob. 1.62PCh. 1 - Draw in all the carbon and hydrogen atoms in each...Ch. 1 - Prob. 1.64PCh. 1 - Prob. 1.65PCh. 1 - Prob. 1.66PCh. 1 - Prob. 1.67PCh. 1 - Each of the following condensed or skeletal...Ch. 1 - Prob. 1.69PCh. 1 - Prob. 1.70PCh. 1 - Prob. 1.71PCh. 1 - Prob. 1.72PCh. 1 - Prob. 1.73PCh. 1 - Prob. 1.74PCh. 1 - Two useful organic compounds that contain Cl atoms...Ch. 1 - Use the symbols + and to indicate the polarity of...Ch. 1 - Label the polar bonds in each molecule. Indicate...Ch. 1 - Answer the following questions about acetonitrile...Ch. 1 - Prob. 1.79PCh. 1 - The principles of this chapter can be applied to...Ch. 1 -
a. What is the hybridization of each N atom in...Ch. 1 - 1.77 Stalevo is the trade name for a medication...Ch. 1 - 1.78 and are two highly reactive carbon...Ch. 1 - 1.79 The N atom in (acetamide) is hybridized,...Ch. 1 - Prob. 1.85PCh. 1 - Prob. 1.86PCh. 1 - Prob. 1.87PCh. 1 - Prob. 1.88PCh. 1 - Prob. 1.89PCh. 1 - Prob. 1.90P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Condensation polymers are produced when monomers containing two different functional groups link together with the loss of a small molecule such as H2O. The difunctional monomer H2N(CH2)6COOH forms a condensation polymer. Draw the carbon-skeleton structure of the dimer that forms from this monomer.arrow_forwardWhat is the structure of the monomer?arrow_forward→ BINDERIYA GANBO... BINDERIYA GANBO. AP Biology Notes Gamino acid chart - G... 36:22 司 10 ☐ Mark for Review Q 1 Hide 80 8 2 =HA O=A¯ = H₂O Acid HIO HBrO HCIO Question 10 of 35 ^ Σ DELL □ 3 % Λ & 6 7 * ∞ 8 do 5 $ 4 # m 3 ° ( 9 Highlights & Notes AXC Sign out Carrow_forward
- Which representation(s) show polymer structures that are likely to result in rigid, hard materials and those that are likely to result in flexible, stretchable, soft materials?arrow_forward3. Enter the molecular weight of the product obtained from the Williamson Ether Synthesis? OH OH & OH excess CH3l Ag₂Oarrow_forwardPlease answer 1, 2 and 3 on the endarrow_forward
- In the box below, specify which of the given compounds are very soluble in polar aprotic solvents. You may select more than one compound. Choose one or more: NaCl NH4Cl CH3CH2CH2CH2CH2CN CH3CH2OH hexan-2-one NaOH CH3SCH3arrow_forwardOn the following structure, select all of the atoms that could ACCEPT a hydrogen bond. Ignore possible complications of aromaticity. When selecting be sure to click on the center of the atom.arrow_forwardRank the compounds below from lowest to highest melting point.arrow_forward
- 18 Question (1 point) Draw the line structure form of the given partially condensed structure in the box provided. :ÖH HC HC H2 ΙΩ Н2 CH2 CH3 CH3 partially condensed formarrow_forwardsomeone else has already submitted the same question on here and it was the incorrect answer.arrow_forwardThe reaction: 2NO2(g) ⇌ N2O4(g) is an exothermic reaction, ΔH=-58.0 kJ/molrxn at 0°C the KP is 58.If the initial partial pressures of both NO2(g) and N2O4(g) are 2.00 atm:A) Is the reaction at equilibrium? If not, what is the value of Q? B) Which direction will the reaction go to reach equilibrium? C) Use an ICE table to find the equilibrium pressures.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License