
The Greenhouse Effect and Global Warming
Although seasons come and go, on average the earth’s climate is very steady. To maintain this stability, the earth must radiate thermal energy—electromagnetic waves—back into space at exactly the same average rate that it receives energy from the sun. Because the earth is much cooler than the sun, its thermal radiation is long-wavelength infrared radiation that we cannot see. A straightforward calculation using Stefan's law finds that the average temperature of the earth should be –18°C, or 0°F, for the incoming and outgoing radiation to lie in balance.
This result is clearly not correct; at this temperature, the entire earth would be covered in snow and ice. The measured global average temperature is actually a balmier 15°C, or 59°F. The straightforward calculation fails because it neglects to consider the earth’s atmosphere. At visible wavelengths, as the figure shows, the atmosphere has a wide “window” of transparency, but this is not true at the infrared wavelengths of the earth’s thermal radiation. The atmosphere lets in the visible radiation from the sun, but the outgoing thermal radiation from the earth sees a much smaller “window.” Most of this radiation is absorbed in the atmosphere.
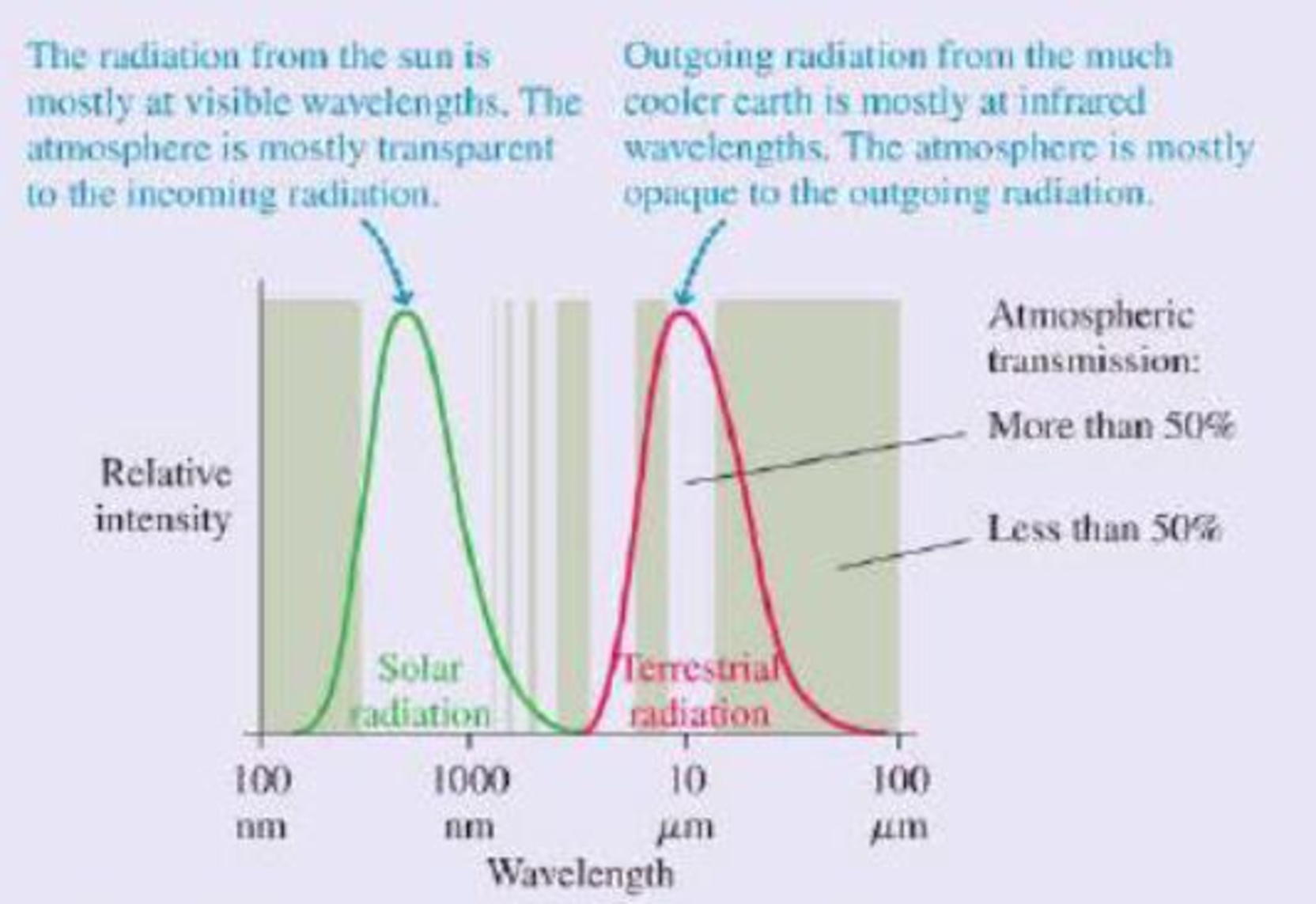
Thermal radiation curves for the sun and the earth. The shaded bands show regions for which the atmosphere is transparent (no shading) or opaque (shaded) to electromagnetic radiation.
Because it’s easier for visible radiant energy to get in than for infrared to get out, the earth is warmer than it would be without the atmosphere. The additional warming of the earth’s surface because of the atmosphere is called the greenhouse effect. The greenhouse effect is a natural part of the earth’s physics; it has nothing to do with human activities, although it’s doubtful any advanced life forms would have evolved without it.
The atmospheric gases most responsible for the greenhouse effect are carbon dioxide and water vapor, both strong absorbers of infrared radiation. These greenhouse gases are of concern today because humans, through the burning of fossil fuels (oil, coal, and natural gas), are rapidly increasing the amount of carbon dioxide in the atmosphere. Preserved air samples show that carbon dioxide made up 0.027% of the atmosphere before the industrial revolution. In the last 150 years, human activities have increased the amount of carbon dioxide by nearly 50%, to about 0.040%. By 2050, the carbon dioxide concentration will likely increase to 0.054%, double the pre-industrial value, unless the use of fossil fuels is substantially reduced.
Carbon dioxide is a powerful absorber of infrared radiation. And good absorbers are also good emitters. The carbon dioxide in the atmosphere radiates energy back to the surface of the earth, warming it. Increasing the concentration of carbon dioxide in the atmosphere means more radiation: this increases the average surface temperature of the earth. The net result is global warming.
There is strong evidence that (he earth has warmed nearly 1°C in the last 100 years because of increased greenhouse gases. What happens next? Climate scientists, using sophisticated models of the earth’s atmosphere and oceans, calculate that a doubling of the carbon dioxide concentration will likely increase the earth’s average temperature by an additional 2°C (≈ 3°F) to 6°C (≈9°F) There is some uncertainty in these calculations; the earth is a large and complex system. Perhaps the earth will get cloudier as the temperature increases, moderating the increase. Or perhaps the arctic ice cap will melt, making the earth less reflective and leading to an even more dramatic
But the basic physics that leads to the greenhouse effect, and to global warming, is quite straightforward. Carbon dioxide in the atmosphere keeps the earth warm; more carbon dioxide will make it warmer. How much warmer? That’s an important question, one that many scientists around the world are attempting to answer with ongoing research. But large or small, change is coming. Global warming is one of the most serious challenges facing scientists, engineers, and all citizens in the 21st century.
The following questions are related to the passage “The Greenhouse Effect and Global Warming” on the previous page.
Electromagnetic waves in certain wavelength ranges interact with water molecules because the molecules have a large electric dipole moment. The electric field of the wave
- A. Exerts a net force on the water molecules.
- B. Exerts a net torque on the water molecules.
- C. Exerts a net force and a net torque on the water molecules.
Want to see the full answer?
Check out a sample textbook solution
Chapter P Solutions
College Physics: A Strategic Approach (3rd Edition)
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Introductory Chemistry (6th Edition)
Chemistry (7th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Campbell Biology in Focus (2nd Edition)
Applications and Investigations in Earth Science (9th Edition)
- Current Attempt in Progress In the figure what is the net electric potential at point P due to the four particles if V = 0 at infinity, q = 2.12 fC, and d = 1.75 cm? d Number MI Units +qarrow_forwardA 0.500 kg sphere moving with a velocity given by (2.00î – 2.60ĵ + 1.00k) m/s strikes another sphere of mass 1.50 kg moving with an initial velocity of (−1.00î + 2.00ĵ – 3.20k) m/s. (a) The velocity of the 0.500 kg sphere after the collision is (-0.90î + 3.00ĵ − 8.00k) m/s. Find the final velocity of the 1.50 kg sphere. R = m/s Identify the kind of collision (elastic, inelastic, or perfectly inelastic). ○ elastic O inelastic O perfectly inelastic (b) Now assume the velocity of the 0.500 kg sphere after the collision is (-0.250 + 0.850ĵ - 2.15k) m/s. Find the final velocity of the 1.50 kg sphere. ✓ = m/s Identify the kind of collision. O elastic O inelastic O perfectly inelastic (c) Take the velocity of the 0.500 kg sphere after the collision as (−1.00ỉ + 3.40] + ak) m/s. Find the value of a and the velocity of the 1.50 kg sphere after an elastic collision. (Two values of a are possible, a positive value and a negative value. Report each with their corresponding final velocities.) a…arrow_forwardA cannon is rigidly attached to a carriage, which can move along horizontal rails, but is connected to a post by a large spring, initially unstretched and with force constant k = 1.31 x 104 N/m, as in the figure below. The cannon fires a 200-kg projectile at a velocity of 136 m/s directed 45.0° above the horizontal. 45.0° (a) If the mass of the cannon and its carriage is 5000 kg, find the recoil speed of the cannon. m/s (b) Determine the maximum extension of the spring. m (c) Find the maximum force the spring exerts on the carriage. (Enter the magnitude of the force.) Narrow_forward
- launch angle. Passage Problems Alice (A), Bob (B), and Carrie (C) all start from their dorm and head for the library for an evening study session. Alice takes a straight path,arrow_forwardbelow the horizontal, and land 55 m horizontally from the end of the jump. Your job is to specify the slope of the ground so skiers' trajectories make an angle of only 3.0° with the ground on land- ing, ensuring their safety. What slope do you specify? T 9.5° -55 marrow_forwardMake sure to draw a sketch and a free body diagram. DO NOT give me examples but ONLY the solutionarrow_forward
- Make sure to draw a sketch AND draw a Free body diagramarrow_forwardP -3 ft 3 ft. O A B 1.5 ft Do 1.5 ft ✓ For the frame and loading shown, determine the magnitude of the reaction at C (in lb) if P = 55 lb. (Hint: Use the special cases: Two-force body and Three-force body.)arrow_forwardA convex mirror (f.=-6.20cm) and a concave minor (f2=8.10 cm) distance of 15.5cm are facing each other and are separated by a An object is placed between the mirrors and is 7.8cm from each mirror. Consider the light from the object that reflects first from the convex mirror and then from the concave mirror. What is the distance of the image (dia) produced by the concave mirror? cm.arrow_forward
- An amusement park spherical mirror shows park spherical mirror shows anyone who stands 2.80m in front of it an upright image one and a half times the person's height. What is the focal length of the minor? m.arrow_forwardAn m = 69.0-kg person running at an initial speed of v = 4.50 m/s jumps onto an M = 138-kg cart initially at rest (figure below). The person slides on the cart's top surface and finally comes to rest relative to the cart. The coefficient of kinetic friction between the person and the cart is 0.440. Friction between the cart and ground can be ignored. (Let the positive direction be to the right.) m M (a) Find the final velocity of the person and cart relative to the ground. (Indicate the direction with the sign of your answer.) m/s (b) Find the friction force acting on the person while he is sliding across the top surface of the cart. (Indicate the direction with the sign of your answer.) N (c) How long does the friction force act on the person? S (d) Find the change in momentum of the person. (Indicate the direction with the sign of your answer.) N.S Find the change in momentum of the cart. (Indicate the direction with the sign of your answer.) N.S (e) Determine the displacement of the…arrow_forwardSmall ice cubes, each of mass 5.60 g, slide down a frictionless track in a steady stream, as shown in the figure below. Starting from rest, each cube moves down through a net vertical distance of h = 1.50 m and leaves the bottom end of the track at an angle of 40.0° above the horizontal. At the highest point of its subsequent trajectory, the cube strikes a vertical wall and rebounds with half the speed it had upon impact. If 10 cubes strike the wall per second, what average force is exerted upon the wall? N ---direction--- ▾ ---direction--- to the top to the bottom to the left to the right 1.50 m 40.0°arrow_forward
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill AstronomyPhysicsISBN:9781938168284Author:Andrew Fraknoi; David Morrison; Sidney C. WolffPublisher:OpenStax
AstronomyPhysicsISBN:9781938168284Author:Andrew Fraknoi; David Morrison; Sidney C. WolffPublisher:OpenStax





