
The Greenhouse Effect and Global Warming
Although seasons come and go, on average the earth’s climate is very steady. To maintain this stability, the earth must radiate thermal energy—electromagnetic waves—back into space at exactly the same average rate that it receives energy from the sun. Because the earth is much cooler than the sun, its thermal radiation is long-wavelength infrared radiation that we cannot see. A straightforward calculation using Stefan's law finds that the average temperature of the earth should be –18°C, or 0°F, for the incoming and outgoing radiation to lie in balance.
This result is clearly not correct; at this temperature, the entire earth would be covered in snow and ice. The measured global average temperature is actually a balmier 15°C, or 59°F. The straightforward calculation fails because it neglects to consider the earth’s atmosphere. At visible wavelengths, as the figure shows, the atmosphere has a wide “window” of transparency, but this is not true at the infrared wavelengths of the earth’s thermal radiation. The atmosphere lets in the visible radiation from the sun, but the outgoing thermal radiation from the earth sees a much smaller “window.” Most of this radiation is absorbed in the atmosphere.
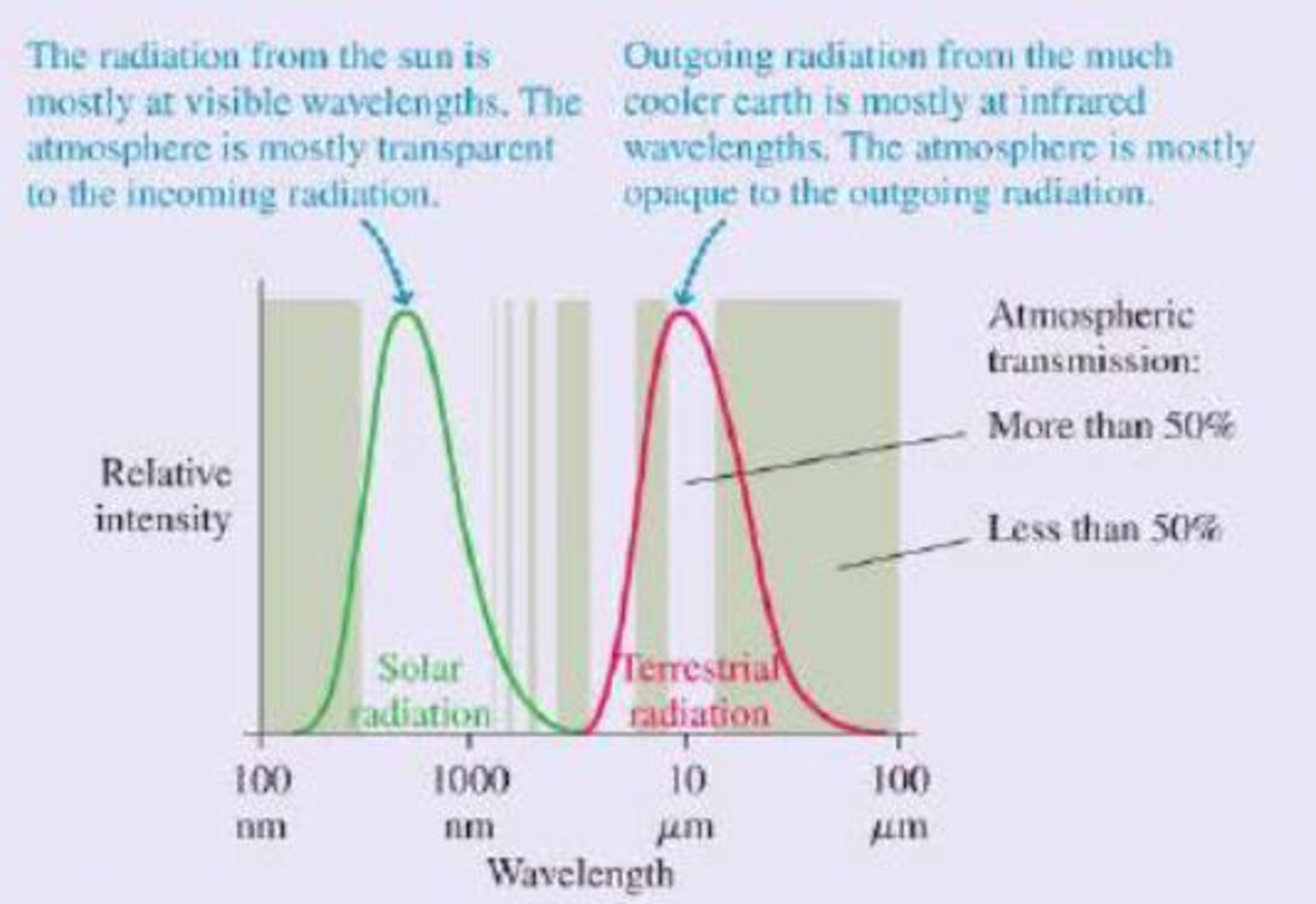
Thermal radiation curves for the sun and the earth. The shaded bands show regions for which the atmosphere is transparent (no shading) or opaque (shaded) to electromagnetic radiation.
Because it’s easier for visible radiant energy to get in than for infrared to get out, the earth is warmer than it would be without the atmosphere. The additional warming of the earth’s surface because of the atmosphere is called the greenhouse effect. The greenhouse effect is a natural part of the earth’s physics; it has nothing to do with human activities, although it’s doubtful any advanced life forms would have evolved without it.
The atmospheric gases most responsible for the greenhouse effect are carbon dioxide and water vapor, both strong absorbers of infrared radiation. These greenhouse gases are of concern today because humans, through the burning of fossil fuels (oil, coal, and natural gas), are rapidly increasing the amount of carbon dioxide in the atmosphere. Preserved air samples show that carbon dioxide made up 0.027% of the atmosphere before the industrial revolution. In the last 150 years, human activities have increased the amount of carbon dioxide by nearly 50%, to about 0.040%. By 2050, the carbon dioxide concentration will likely increase to 0.054%, double the pre-industrial value, unless the use of fossil fuels is substantially reduced.
Carbon dioxide is a powerful absorber of infrared radiation. And good absorbers are also good emitters. The carbon dioxide in the atmosphere radiates energy back to the surface of the earth, warming it. Increasing the concentration of carbon dioxide in the atmosphere means more radiation: this increases the average surface temperature of the earth. The net result is global warming.
There is strong evidence that (he earth has warmed nearly 1°C in the last 100 years because of increased greenhouse gases. What happens next? Climate scientists, using sophisticated models of the earth’s atmosphere and oceans, calculate that a doubling of the carbon dioxide concentration will likely increase the earth’s average temperature by an additional 2°C (≈ 3°F) to 6°C (≈9°F) There is some uncertainty in these calculations; the earth is a large and complex system. Perhaps the earth will get cloudier as the temperature increases, moderating the increase. Or perhaps the arctic ice cap will melt, making the earth less reflective and leading to an even more dramatic
But the basic physics that leads to the greenhouse effect, and to global warming, is quite straightforward. Carbon dioxide in the atmosphere keeps the earth warm; more carbon dioxide will make it warmer. How much warmer? That’s an important question, one that many scientists around the world are attempting to answer with ongoing research. But large or small, change is coming. Global warming is one of the most serious challenges facing scientists, engineers, and all citizens in the 21st century.
The following questions are related to the passage “The Greenhouse Effect and Global Warming” on the previous page.
The intensity of sunlight at the top of the earth’s atmosphere is approximately 1400 W/m2. Mars is about 1.5 times as far from the sun as the earth. What is the approximate intensity of sunlight at the top of Mar’s atmosphere?
- A. 930 W/m2
- B. 620 W/m2
- C. 410 W/m2
- D. 280 W/m2
Want to see the full answer?
Check out a sample textbook solution
Chapter P Solutions
College Physics: A Strategic Approach (3rd Edition)
Additional Science Textbook Solutions
Introductory Chemistry (6th Edition)
Human Biology: Concepts and Current Issues (8th Edition)
Microbiology with Diseases by Body System (5th Edition)
Campbell Biology (11th Edition)
Chemistry (7th Edition)
Campbell Essential Biology with Physiology (5th Edition)
- In the comics Thor flies by spinning his hammer really fast from a leather strap at the end of the handle, letting go, then grabbing it and having it pull him. If Thor wants to reach escape velocity (velocity needed to leave Earth’s atmosphere), he will need the linear velocity of the center of mass of the hammer to be 11,200 m/s. A) If the distance from the end of the strap to the center of the hammer is 0.334 m, what angular velocity does Thor need to spin his hammer at to reach escape velocity? b) If the hammer starts from rest what angular acceleration does Thor need to reach that angular velocity in 4.16 s? c) While the hammer is spinning at its maximum speed what impossibly large tension does the leather strap, which the hammer is spinning by, exert when the hammer is at its lowest point? The hammer has a total mass of 20.0kg.arrow_forwardThe car goes from driving straight to spinning at 10.6 rev/min in 0.257 s with a radius of 12.2 m. The angular accleration is 4.28 rad/s^2. During this flip Barbie stays firmly seated in the car’s seat. Barbie has a mass of 58.0 kg, what is her normal force at the top of the loop?arrow_forwardConsider a hoop of radius R and mass M rolling without slipping. Which form of kinetic energy is larger, translational or rotational?arrow_forward
- A roller-coaster vehicle has a mass of 571 kg when fully loaded with passengers (see figure). A) If the vehicle has a speed of 22.5 m/s at point A, what is the force of the track on the vehicle at this point? B) What is the maximum speed the vehicle can have at point B, in order for gravity to hold it on the track?arrow_forwardThis one wheeled motorcycle’s wheel maximum angular velocity was about 430 rev/min. Given that it’s radius was 0.920 m, what was the largest linear velocity of the monowheel?The monowheel could not accelerate fast or the rider would start spinning inside (this is called "gerbiling"). The maximum angular acceleration was 10.9 rad/s2. How long, in seconds, would it take it to hit maximum speed from rest?arrow_forwardIf points a and b are connected by a wire with negligible resistance, find the magnitude of the current in the 12.0 V battery.arrow_forward
- Consider the two pucks shown in the figure. As they move towards each other, the momentum of each puck is equal in magnitude and opposite in direction. Given that v kinetic energy of the system is converted to internal energy? 30.0° 130.0 = green 11.0 m/s, and m blue is 25.0% greater than m 'green' what are the final speeds of each puck (in m/s), if 1½-½ t thearrow_forwardConsider the blocks on the curved ramp as seen in the figure. The blocks have masses m₁ = 2.00 kg and m₂ = 3.60 kg, and are initially at rest. The blocks are allowed to slide down the ramp and they then undergo a head-on, elastic collision on the flat portion. Determine the heights (in m) to which m₁ and m2 rise on the curved portion of the ramp after the collision. Assume the ramp is frictionless, and h 4.40 m. m2 = m₁ m hm1 hm2 m iarrow_forwardA 3.04-kg steel ball strikes a massive wall at 10.0 m/s at an angle of 0 = 60.0° with the plane of the wall. It bounces off the wall with the same speed and angle (see the figure below). If the ball is in contact with the wall for 0.234 s, what is the average force exerted by the wall on the ball? magnitude direction ---Select--- ✓ N xarrow_forward
- You are in the early stages of an internship at NASA. Your supervisor has asked you to analyze emergency procedures for extravehicular activity (EVA), when the astronauts leave the International Space Station (ISS) to do repairs to its exterior or perform other tasks. In particular, the scenario you are studying is a failure of the manned-maneuvering unit (MMU), which is a nitrogen-propelled backpack that attaches to the astronaut's primary life support system (PLSS). In this scenario, the astronaut is floating directly away from the ISS and cannot use the failed MMU to get back. Therefore, the emergency plan is to take off the MMU and throw it in a direction directly away from the ISS, an action that will hopefully cause the astronaut to reverse direction and float back to the station. You have the following mass data provided to you: astronaut: 78.1 kg, spacesuit: 36.8 kg, MMU: 115 kg, PLSS: 145 kg. Based on tests performed by astronauts floating "weightless" inside the ISS, the most…arrow_forwardThree carts of masses m₁ = 4.50 kg, m₂ = 10.50 kg, and m3 = 3.00 kg move on a frictionless, horizontal track with speeds of V1 v1 13 m 12 mq m3 (a) Find the final velocity of the train of three carts. magnitude direction m/s |---Select--- ☑ (b) Does your answer require that all the carts collide and stick together at the same moment? ○ Yes Ο Νο = 6.00 m/s to the right, v₂ = 3.00 m/s to the right, and V3 = 6.00 m/s to the left, as shown below. Velcro couplers make the carts stick together after colliding.arrow_forwardA girl launches a toy rocket from the ground. The engine experiences an average thrust of 5.26 N. The mass of the engine plus fuel before liftoff is 25.4 g, which includes fuel mass of 12.7 g. The engine fires for a total of 1.90 s. (Assume all the fuel is consumed.) (a) Calculate the average exhaust speed of the engine (in m/s). m/s (b) This engine is positioned in a rocket body of mass 70.0 g. What is the magnitude of the final velocity of the rocket (in m/s) if it were to be fired from rest in outer space with the same amount of fuel? Assume the fuel burns at a constant rate. m/sarrow_forward
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill AstronomyPhysicsISBN:9781938168284Author:Andrew Fraknoi; David Morrison; Sidney C. WolffPublisher:OpenStax
AstronomyPhysicsISBN:9781938168284Author:Andrew Fraknoi; David Morrison; Sidney C. WolffPublisher:OpenStax





