
Concept explainers
a)
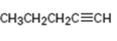
Interpretation:
How to synthesize 1-pentyne from acetylene using any alkyl halide with four or fewer number of carbons is to be shown.
Concept introduction:
Terminal
To state:
How to synthesize 1-pentyne from acetylene using any alkyl halide with four or fewer number of carbons.
b)
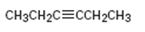
Interpretation:
How to synthesize 3-hexyne from acetylene using any alkyl halide with four or fewer number of carbons is to be shown.
Concept introduction:
Terminal alkynes can be converted into their alkynides by treating with NaNH3 in liquid NH3. The alkynides when treated with alkyl halides with the required number of carbons yield the higher alkyne needed. Acetylene has two acidic hydrogens. Both hydrogens can be replaced by alkyl groups through this process.
To state:
How to synthesize 3-hexyne from acetylene using any alkyl halide with four or fewer number of carbons.
c)
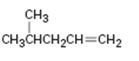
Interpretation:
How to synthesize 4-methyl-1-pentene from acetylene using any alkyl halide with four or fewer number of carbons is to be shown.
Concept introduction:
Terminal alkynes can be converted into their alkynides by treating with NaNH3 in liquid NH3. The alkynides when treated with alkyl halides with the required number of carbons yield the higher alkyne needed. The alkyne can be reduced to the corresponding
To state:
How to synthesize 4-methyl-1-pentene from acetylene using any alkyl halide with four or fewer number of carbons.
d)
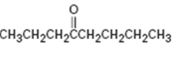
Interpretation:
How to synthesize 4-octanone from acetylene using any alkyl halide with four or fewer number of carbons is to be shown.
Concept introduction:
Terminal alkynes can be converted into their alkynides by treating with NaNH3 in liquid NH3. The alkynides when treated with alkyl halides with the required number of carbons yield the higher alkyne needed. The alkyne undergoes hydration when treated with dilute H3SO4 in the presence of HgSO4 to yield an enol which tautomerizes to a
To state:
How to synthesize 4-octanone from acetylene using any alkyl halide with four or fewer number of carbons.
e)
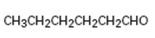
Interpretation:
How to synthesize hexanal from acetylene using any alkyl halide with four or fewer number of carbons is to be shown.
Concept introduction:
Terminal alkynes can be converted into their alkynides by treating with NaNH3 in liquid NH3. The alkynides when treated with alkyl halides with the required number of carbons yield the higher alkynes needed. The alkynes yield enols with OH on terminal carbon in hydroboration-oxidation reaction which tautomerize to yield
To state:
How to synthesize hexanal from acetylene using any alkyl halide with four or fewer number of carbons.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- Problem 54, could you please explain it in detail? Thank you! Step by step, I'm really confused, so please don't make it overly complex. My question is to visually draw it out and demonstrate it to me; I'm confused about that problem, please (not just in words) but demonstrate it to me in all due essence (visually) with descriptions.arrow_forwardExplain the types of electromeric effects +E and -E.arrow_forwardBriefly describe the electromeric effect (Organic Chemistry)arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. (CH3)2NH, TSOH Drawingarrow_forwardSo, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forward
- Draw a mental model for calcium chloride mixed with sodium phosphatearrow_forwardhere is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

