
a)
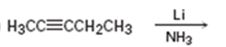
Interpretation:
The product formed and the electron pushing mechanism for its formation, when 2-pentyne is reduced with Li in NH3 is to be given.
Concept introduction:
Lithium metal donates an electron to the
The addition takes place with trans stereochemistry. The stereochemistry is established in the third step when the less hindered vinylic anion is formed from vinylic radical.
To propose:
The product formed and the electron pushing mechanism for its formation, when 2-pentyne is reduced with Li in NH3.
b)
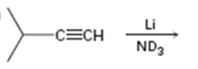
Interpretation:
The product formed and the electron pushing mechanism for its formation, when 3-methyl-1-butyne is reduced with Li in ND3 is to be given.
Concept introduction:
Lithium metal donates an electron to the alkyne to give an anion radical which in the next step abstracts a proton from deuteratedammonia solvent to yield a vinylic radical. In the third step the vinylic radical accepts a second electron from another Li atom to produce a vinylic anion which in the fourth step abstracts another proton from the solvent to yield the final trans product.
The addition takes place with trans stereochemistry. The stereochemistry is established in the third step when the less hindered vinylic anion is formed from vinylic radical.
To propose:
The product formed and the electron pushing mechanism for its formation, when 3-methyl-1-butyne is reduced with Li in ND3.
c)
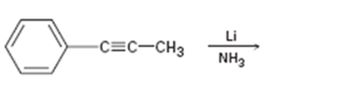
Interpretation:
The product formed and the electron pushing mechanism for its formation, when propynylbenzene is reduced with Li in NH3 is to be given.
Concept introduction:
Lithium metal donates an electron to the alkyne to give an anion radical which in the next step abstracts a proton from ammonia solvent to yield a vinylic radical. In the third step the vinylic radical accepts a second electron from another Li atom to produce a vinylic anion which in the fourth step abstracts another proton from ammonia solvent to yield the final trans product.
The addition takes place with trans stereochemistry. The stereochemistry is established in the third step when the less hindered vinylic anion is formed from vinylic radical.
To propose:
The product formed and the electron pushing mechanism for its formation, when propynylbenzene is reduced with Li in NH3.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- The inductive effect (+I and -I) in benzene derivatives, does it guide ortho, meta or para?arrow_forward19.57 Using one of the reactions in this chapter, give the correct starting material (A-L) needed to produce each structure (a-f). Name the type of reaction used. (b) ہ مرد (d) HO (c) དང་ ་་ཡིན་ད་དང་ (f) HO Br B D of oli H J Br K C 人 ↑arrow_forwardInductive effect (+I and -I) in benzene derivatives.arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


