
a)

Interpretation:
How to convert 1-butyne in to butanone is to be shown.
Concept introduction:
Terminal
To show:
How to convert 1-butyne in to butanone.
b)
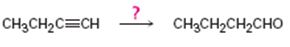
Interpretation:
How to convert 1-butyne in to butanal is to be shown.
Concept introduction:
Terminal alkynes when hydrated using hydroboration-oxidation reaction yield an enol with OH group attached to the terminal carbon as the reaction follows anti-Markovnikov regiochemistry. The enol obtained then tautomerizes to an
To show:
How to convert 1-butyne in to butanal is to be shown.
c)
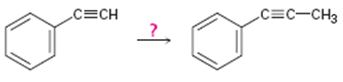
Interpretation:
How to convert ethynylbenzene in to propynylbenzene is to be shown.
Concept introduction:
Terminal alkynes are converted in to acetylides by treating with NaNH2 in liquid ammonia. The acetylides can be converted to higher alkynes by treating with
To show:
How to convert ethynylbenzene in to propynylbenzene.
d)
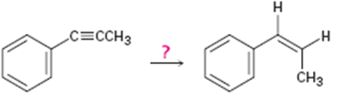
Interpretation:
How to convert propynylbenzene in to cis-1-propenylbenzene is to be shown.
Concept introduction:
Alkynes can be converted in to the corresponding
To show:
How to convert 1-propynylbenzene in to 1-propenylbenzene.
e)

Interpretation:
How to convert 1-butyne in to propanoic acid is to be shown.
Concept introduction:
Alkynes gets cleaved when treated with powerful oxidizing agents like KMnO4. Internal alkynes give carboxylic acids as products. Terminal alkynes give CO2 also along with a
To show:
How to convert 1-butyne in to propanoic acid.
f)
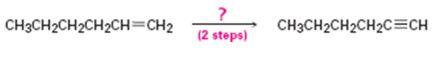
Interpretation:
How to convert 1-hexene in to 1-hexyne in two steps is to be shown.
Concept introduction:
Alkenes when treated with bromine yield a dibromo
To show:
How to convert 1-hexene in to 1-hexyne in two steps
Trending nowThis is a popular solution!

Chapter 9 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- The inductive effect (+I and -I) in benzene derivatives, does it guide ortho, meta or para?arrow_forward19.57 Using one of the reactions in this chapter, give the correct starting material (A-L) needed to produce each structure (a-f). Name the type of reaction used. (b) ہ مرد (d) HO (c) དང་ ་་ཡིན་ད་དང་ (f) HO Br B D of oli H J Br K C 人 ↑arrow_forwardInductive effect (+I and -I) in benzene derivatives.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

