
a)
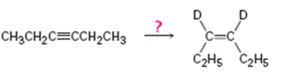
Interpretation:
How to carry out the reaction given which yields deuterium incorporated alkene as the product is to be shown.
Concept introduction:
Deuterium incorporated
To show:
How to carry out the reaction given which yields deuterium incorporated alkene as the product.
b)
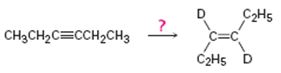
Interpretation:
How to carry out the reaction given which yields deuterium incorporated alkene as the product is to be shown.
Concept introduction:
Deuterium incorporated alkenes can be prepared from alkynes by reduction in the presence of catalysts. Use of deuterium in the presence of Lindlar catalyst yields cis alkenes with the two deuterium atoms arranged on the same side of the double bond while reduction with Li in liquid deuterated ammonia yields trans alkenes with the two deuterium atoms arranged on the opposite sides of the double bond.
To show:
How to carry out the reaction given which yields deuterium incorporated alkene as the product.
c)
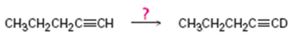
Interpretation:
How to carry out the reaction given which yields deuterium incorporated alkyne as the product is to be shown.
Concept introduction:
Deuterium incorporated alkynes can be prepared first by converting them in to alkynides by treating with NaNH2 in NH3 and then treating the alkynide obtained with D3O+.
To show:
How to carry out the reaction given which yields deuterium incorporated alkyne as the product.
d)
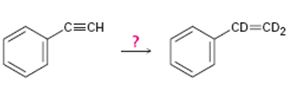
Interpretation:
How to carry out the reaction given which yields deuterium incorporated alkene as the product is to be shown.
Concept introduction:
Deuterium incorporated alkynes can be prepared first by converting them in to alkynides by treating with NaNH2 in NH3 and then treating the alkynide obtained with D3O+. The alkyne thus obtained when treated with deuterium in the presence of Lindlar catalyst yield an alkene with deuterium atom on both carbons.
To show:
How to carry out the reaction given which yields deuterium incorporated alkene as the product.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- Rank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forwardCould you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forward
- What are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forwardPlease use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forward
- 3. Predict the major product and give a mechanism for the following reactions: (CH3)3COH/H₂SO4 a) b) NC CH₂O c) LOCH, (CH3)3COH/H2SO4 H,SO -OHarrow_forwardIndicate if the aldehyde shown reacts with the provided nucleophiles in acid or base conditions. a NaBH4 be Li eli -NH2 P(Ph3) f KCN g OH excess h CH3OH i NaCHCCH3arrow_forwardPredict the major products of the following organic reaction: + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole



