
a)
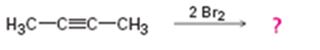
Interpretation:
Assuming that halogens add to
Concept introduction:
Alkynes when treated with one equivalent of a halogen yield a dihaloalkene as the product. They react with two equivalents of the halogens to yield a tetrahaloalkane derivative. In the first step of the addition reaction the nucleophilic attack of the π electrons of the double/triple bond in alkene/alkyne on a halogen results in the formation of a cyclic halonium ion with the simultaneous elimination of a halide ion. In the second step the halide ion attacks the cyclic halonium ion to yield the product.
To propose:
A mechanism and to predict the product(s) expected for the reaction in which two equivalents of Br2 adds to 2-butyne assuming that bromine adds to alkynes in the same manner as they add to alkenes.
b)
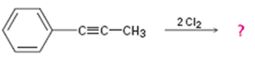
Interpretation:
Assuming that halogens add to alkynes in the same manner as they add to alkenes, a mechanism is to be proposed and the product(s) expected for the reaction in which two equivalents of Cl2 adds to 1-phenylpropyne is/are to be predicted.
Concept introduction:
Alkynes when treated with one equivalent of a halogen yield a dihaloalkene as the product. They react with two equivalents of the reagents to yield a tetrahaloalkane as the product. In the first step of the addition reaction, the nucleophilic attack of the π electrons of the double/triple bond in alkene/alkyne on a halogen results in the formation of a cyclic halonium ion with the simultaneous elimination of a halide ion. In the second step the halide ion attacks the cyclic halonium ion to yield the product.
To propose:
A mechanism and to predict the product(s) expected for the reaction in which two equivalents of Cl2 adds to 1-phenylpropyne assuming that chlorine adds to alkynes in the same manner as they add to alkenes.
c)
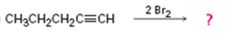
Interpretation:
Assuming that halogens add to alkynes in the same manner as they add to alkenes, a mechanism is to be proposed and the product(s) expected for the reaction in which two equivalents of Br2 adds to 1-pentyne is/are to be predicted.
Concept introduction:
Alkynes when treated with one equivalent of a halogen yield a dihaloalkene as the product. They react with two equivalents of the reagents to yield a tetrahaloalkane as the product. In the first step of the addition reaction the nucleophilic attack of the π electrons of the double/triple bond in alkene/alkyne on a halogen results in the formation of a cyclic halonium ion with the simultaneous elimination of a halide ion. In the second step the halide ion attacks the cyclic halonium ion to yield the product.
To propose:
A mechanism and to predict the product(s) expected for the reaction in which two equivalents of Br2 adds to 1-pentyne assuming that bromine adds to alkynes in the same manner as they add to alkenes.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- So, the first image is what I'm trying to understand regarding my approach. The second image illustrates my teacher's method, and the third image includes my notes on the concepts behind these types of problems.arrow_forwardHAND DRAWarrow_forwardDraw a mental model for calcium chloride mixed with sodium phosphatearrow_forward
- here is my question (problem number 20) please explain to me thanks!arrow_forwardThe bromination of anisole is an extremely fast reaction. Complete the resonance structures of the intermediate arenium cation for the reaction (Part 1), and then answer the question that follows (Part 2).arrow_forwardDrawing of 3-fluro-2methylphenolarrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole




