
Concept explainers
Name the following
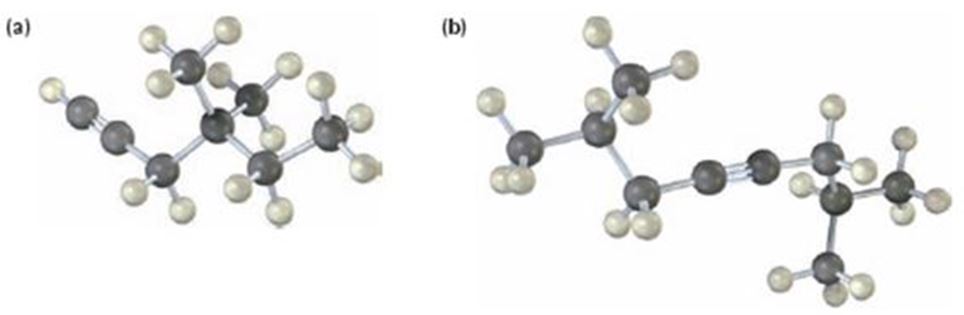
a)
Interpretation:
The alkyne shown is to be named and the products formed when it reacts with 1) H2 in the presence of Lindlar catalyst and 2) H3O+ in the presence of HgSO4 is to be predicted.
Concept introduction:
The longest carbon chain which contains the carbon-carbon triple bond is chosen. The chain is numbered from the end that gives the lowest number to the carbon in triple bond. Compounds with more than one triple bond are called diynes, triynes and so forth. The substituents present, if any are written in the alphabetical order.
When reduced with Hydrogen in the presence of Lindlar catalyst the reduction of alkynes stops in the alkene stage. When treated with H3O+ in the presence of HgSO4, alkynes undergo hydration following Markovnikov regiochemistry to give an enols which will tautomerize to yield aldehydes (terminal alkynes) or ketones (internal alkynes).
To give:
The name of the alkyne shown and to predict the products formed when it reacts with 1) H2 in the presence of Lindlar catalyst and 2) H3O+ in the presence of HgSO4.
Answer to Problem 14VC
The name of the alkyne shown is 4,4-dimethyl-1-hexyne.
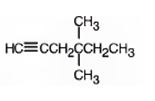
The product formed when it reacts with H2 in the presence of Lindlar catalyst is 4,4-dimethyl-1-hexene.
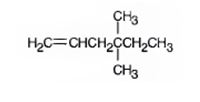
The product formed when it reacts with H3O+ in the presence of HgSO4 is 4,4-dimethyl-2-hexanone.
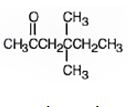
Explanation of Solution
The compound has a six carbon straight chain with two methyl groups on C4 with a triple bond between C1 & C2. Hence it’s name is 4,4-dimethyl-1-hexyne.
When reduced with hydrogen in the presence of Lindlar catalyst, the triple bond becomes a double bond as each of the two carbons in the triple bond gets attached to a hydrogen and an alkene, 4,4-dimethyl-1-hexene,is thus produced.
When treated with H3O+ in the presence of HgSO4, the addition of water takes place in the triple bond following Markovnikov regiochemistry. The OH group adds to more highly substituted carbon and H adds to the less highly substituted carbon in triple bond resulting in the formation of an enol which undergoes tautomerization to yield the ketone, 4,4-dimethyl-2-hexanone.
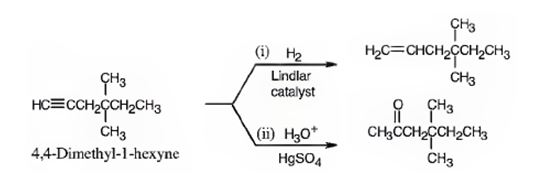
The name of the alkyne shown is 4,4-dimethyl-1-hexyne.

The product formed when it reacts with H2 in the presence of Lindlar catalyst is 4,4-dimethyl-1-hexene.

The product formed when it reacts with H3O+ in the presence of HgSO4 is 4,4-dimethyl-2-hexanone.

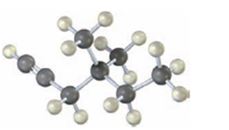
b)
Interpretation:
The alkyne shown is to be named and the products formed when it reacts with 1) H2 in the presence of Lindlar catalyst and 2) H3O+ in the presence of HgSO4 is to be predicted.
Concept introduction:
The longest carbon chain which contains the carbon-carbon triple bond is chosen. The chain is numbered from the end that gives the lowest number to the carbon in triple bond. Compounds with more than one triple bond are called diynes, triynes and so forth. The substituents present, if any are written in the alphabetical order.
When reduced with Hydrogen in the presence of Lindlar catalyst the reduction of alkynes stops in the alkene stage. When treated with H3O+ in the presence of HgSO4, alkynes undergo hydration following Markovnikov regiochemistry to give an enols which will tautomerize to yield aldehydes (terminal alkynes) or ketones (internal alkynes).
To give:
The name of the alkyne shown and to predict the products formed when it reacts with 1) H2 in the presence of Lindlar catalyst and 2) H3O+ in the presence of HgSO4.
Answer to Problem 14VC
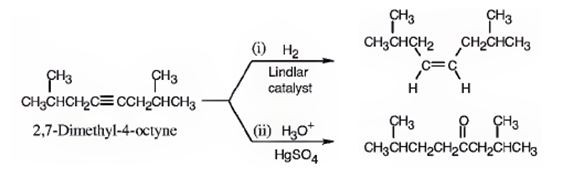
The name of the alkyne shown is 2,7-dimethyl-4-octyne.
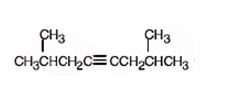
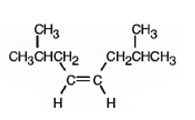
The product formed when it reacts with H2 in the presence of Lindlar catalyst is cis-2,7-dimethyl-4-octene.
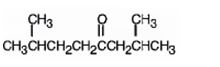
The product formed when it reacts with H3O+ in the presence of HgSO4 is 2,7-dimethyl-4-octanone.
Explanation of Solution
The compound has an eight carbon straight chain with two methyl groups on C2 & C7 with a triple bond between C4 & C5. Hence it’s name is 2,7-dimethyl-4-octyne.
When reduced with hydrogen in the presence of Lindlar catalyst, the triple bond becomes a double bond as each of the two carbons in the triple bond gets attached to a hydrogen and an alkene, cis- 2,7-dimethyl-4-octene.is thus produced.
When treated with H3O+ in the presence of HgSO4, the addition of water takes place in the triple bond. As the alkene is symmetrical the Markovnikov regiochemistry cannot be applied. The OH group adds to one carbon and H adds to the other carbon in triple bond resulting in the formation of an enol which undergoes tautomerization to yield the ketone, 2,7-dimethyl-4-octanone.
The name of the alkyne shown is 2,7-dimethyl-4-octyne.

The product formed when it reacts with H2 in the presence of Lindlar catalyst is cis-2,7-dimethyl-4-octene.

The product formed when it reacts with H3O+ in the presence of HgSO4 is 2,7-dimethyl-4-octanone.

Want to see more full solutions like this?
Chapter 9 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- Please label this COZY spectraarrow_forwardPlease label this HNMRarrow_forwardConsider the following gas chromatographs of Compound A, Compound B, and a mixture of Compounds A and B. Inject A B mixture Area= 9 Area = 5 Area = 3 Area Inject . མི། Inject J2 What is the percentage of Compound B in the the mixture?arrow_forward
- Rank these according to stability. CH3 H3C CH3 1 CH3 H3C 1 most stable, 3 least stable O 1 most stable, 2 least stable 2 most stable, 1 least stable O2 most stable, 3 least stable O3 most stable, 2 least stable O3 most stable, 1 least stable CH3 2 CH3 CH3 H₂C CH3 3 CH3 CHarrow_forwardConsider this IR and NMR: INFRARED SPECTRUM TRANSMITTANCE 0.8- 0.6 0.4 0.2 3000 10 9 8 00 HSP-00-541 7 CO 6 2000 Wavenumber (cm-1) сл 5 ppm 4 M Which compound gave rise to these spectra? N 1000 1 0arrow_forwardConsider this reaction (molecular weights are under each compound): HC=CH + 2 HCI --> C2H4Cl 2 MW = 26 36.5 99 If 4.4 g of HC=CH are reacted with 110 mL of a 2.3 M HCI solution, and 6.0 g of product are actually produced, what is the percent yield?arrow_forward
- What is the name of the major product of this reaction? OH CH3 H₂SO4, heat 1-methylcyclohexene O2-methyl-1-cyclohexene O 3-mthylcyclohexene 1-methyl-2-cyclohexenearrow_forwardWe added a brown solution of Br2 to one of our products, and the brown color disappeared. This indicated that our product wasarrow_forwardRank the following according to reactivity toward nitration: a) benzene b) bromobenzene c) nitrobenzene d) phenol Od) greatest, c) least Od) greatest, b) least Od) greatest, a) least a) greatest, b) least a) greatest, c) least Oa) greatest, d) least Ob) greatest, a) least O b) greatest, c) least Ob) greatest, d) least O c) greatest, a) least O c) greatest, b) least O c) greatest, d) leastarrow_forward
- O-Nitrophenol was distilled over with the steam in our experiment while the other isomer did not. This is due to: O intramolecular hydrogen bonding in the ortho isomer O intermolecular hydrogen bonding in the the ortho isomer O the ortho isomer has a lower density O the ortho isomer has a lower molecular weightarrow_forwardK 44% Problem 68 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :6: :: :CI: CI CI: :0:0 Select to Add Arrows Select to Add Arrows H H Cl CI: CI CI: Select to Add Arrows Select to Add Arrows H :CI: Alarrow_forwardI I H :0: Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. 0:0 :0: CI ΑΙ :CI: :CI: :0: CI Select to Add Arrows Select to Add Arrows cl. :0: Cl © ハ CI:: CI H CO Select to Add Arrows Select to Add Arrows 10: AI ::arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

