
Concept explainers
(a)
Interpretation:
The major product of the given reaction should be drawn.
Concept introduction:
Ring-opening of
Acid-catalyzed ring-opening of epoxide: The epoxide ring is protonated and the nucleophile attack depends on the electronic or steric effect (nature of epoxide).
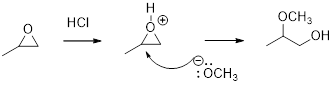
Regiochemistry: When the epoxide is unsymmetrical, the nucleophile attack at the more substituted position of the protonated epoxide ring.
Stereochemistry: when the nucleophile attack takes place at chiral center, an inversion of configuration is obtained.
Base catalyzed ring opening of epoxide:
The nucleophile will attack at the less substituted position under basic conditions and then the alkoxide ion gets proton from alcohol which form the product.
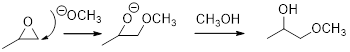
(b)
Interpretation:
The major product of the given reaction should be drawn.
Concept introduction:
Ring-opening of epoxide: The epoxide ring is highly strain and readily undergoes reaction with strong nucleophile result in the ring-opening reaction.
Acid-catalyzed ring-opening of epoxide: The epoxide ring is protonated and the nucleophile attack depends on the electronic or steric effect (nature of epoxide).

Regiochemistry: When the epoxide is unsymmetrical, the nucleophile attack at the more substituted position of the protonated epoxide ring.
Stereochemistry: when the nucleophile attack takes place at chiral center, an inversion of configuration is obtained.
Base catalyzed ring opening of epoxide:
The nucleophile will attack at the less substituted position under basic conditions and then the alkoxide ion gets proton from alcohol which form the product.
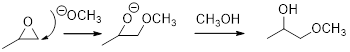
(c)
Interpretation:
The major product of the given reaction should be drawn.
Concept introduction:
Ring-opening of epoxide: The epoxide ring is highly strain and readily undergoes reaction with strong nucleophile result in the ring-opening reaction.
Acid-catalyzed ring-opening of epoxide: The epoxide ring is protonated and the nucleophile attack depends on the electronic or steric effect (nature of epoxide).

Regiochemistry: When the epoxide is unsymmetrical, the nucleophile attack at the more substituted position of the protonated epoxide ring.
Stereochemistry: when the nucleophile attack takes place at chiral center, an inversion of configuration is obtained.
Base catalyzed ring opening of epoxide:
The nucleophile will attack at the less substituted position under basic conditions and then the alkoxide ion gets proton from alcohol which form the product.
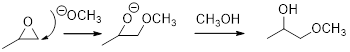
(d)
Interpretation:
The major product of the given reaction should be drawn.
Concept introduction:
Ring-opening of epoxide: The epoxide ring is highly strain and readily undergoes reaction with strong nucleophile result in the ring-opening reaction.
Acid-catalyzed ring-opening of epoxide: The epoxide ring is protonated and the nucleophile attack depends on the electronic or steric effect (nature of epoxide).

Regiochemistry: When the epoxide is unsymmetrical, the nucleophile attack at the more substituted position of the protonated epoxide ring.
Stereochemistry: when the nucleophile attack takes place at chiral center, an inversion of configuration is obtained.
Base catalyzed ring opening of epoxide:
The nucleophile will attack at the less substituted position under basic conditions and then the alkoxide ion gets proton from alcohol which form the product.
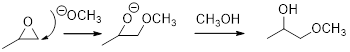
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Essential Organic Chemistry, Global Edition
- Q1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardIndicate which of the following is not an element in its standard state at 25oC and 1 atm. Group of answer choices O2(g) H2(g) Ne(g) N(g) C(s, graphite)arrow_forward6. Show how you would accomplish the following transformations. (Show the steps and reagents/solvents needed) 2-methylpropene →2,2-dimethyloxiran Iarrow_forward
- 4) Answer the following exercise with curved arrows indicating who is a nucleophile or Who is the electrophile? 2.44 Predict the structure of the product formed in the reaction of the organic base pyridine with the organic acid acetic acid, and use curved arrows to indicate the direction of electron flow. 7 H3C OH N Pyridine Acetic acidarrow_forwardUsing the data provided please help me answer this question. Determine the concentration of the iron(Ill) salicylate in the unknown directly from to graph and from the best fit trend-line (least squares analysis) of the graph that yielded a straight line.arrow_forwardPlease help me figure out what the slope is and how to calculate the half life Using the data provided.arrow_forward
- Curved arrows are used to illustrate the flow of electrons. Follow the curved arrows and draw the structure of the missing reactants, intermediates, or products in the following mechanism. Include all lone pairs. Ignore stereochemistry. Ignore inorganic byproducts. H Br2 (1 equiv) H- Select to Draw Starting Alkene Draw Major Product I I H2O 四: ⑦.. Q Draw Major Charged Intermediate Iarrow_forwardNH (aq)+CNO (aq) → CO(NH2)2(s) Experiment [NH4] (M) [CNO] (M) Initial rate (M/s) 1 0.014 0.02 0.002 23 0.028 0.02 0.008 0.014 0.01 0.001 Calculate the rate contant for this reaction using the data provided in the table.arrow_forward2CIO2 + 20H-1 CIO31 + CIO2 + H2O Experiment [CIO2], M [OH-1], M 1 0.0500 0.100 23 2 0.100 0.100 3 0.100 0.0500 Initial Rate, M/s 0.0575 0.230 0.115 ... Given this date, calculate the overall order of this reaction.arrow_forward
- 2 3 .(be)_[Ɔ+(be)_OI ← (b²)_IƆO+ (be)_I Experiment [1-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 0.000069 4 0.0025 0.0025 0.000140 Calculate the rate constant of this reaction using the table data.arrow_forward1 2 3 4 I(aq) +OCl(aq) → IO¯¯(aq) + Cl¯(aq) Experiment [I-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 Calculate the overall order of this reaction using the table data. 0.0025 0.000069 0.0025 0.000140arrow_forwardH2O2(aq) +3 I¯(aq) +2 H+(aq) → 13(aq) +2 H₂O(l)· ••• Experiment [H2 O2]o (M) [I]o (M) [H+]。 (M) Initial rate (M/s) 1 0.15 0.15 0.05 0.00012 234 0.15 0.3 0.05 0.00024 0.3 0.15 0.05 0.00024 0.15 0.15 0.1 0.00048 Calculate the overall order of this reaction using the table data.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





