
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 55P
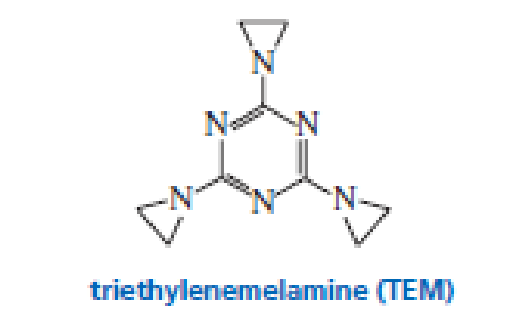
Triethylenemelamine (TEM) is an antitumor agent. Its activity is due to its ability to cross-link DNA
- a. Explain why it can be used only under slightly acidic conditions.
- b. Explain why it can cross-link DNA.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Sho reaction mechanism. Don't give Ai generated solution
Is this aromatic?
finish these to given parts of the reaction then give the final pro
Chapter 9 Solutions
Essential Organic Chemistry, Global Edition
Ch. 9.1 - Draw the structures of straight-chain alcohols...Ch. 9.1 - Prob. 2PCh. 9.1 - Prob. 3PCh. 9.2 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 9.2 - Prob. 5PCh. 9.2 - The observed relative reactivities of primary,...Ch. 9.4 - Which of the following alcohols would dehydrate...Ch. 9.4 - Prob. 10PCh. 9.4 - Prob. 11PCh. 9.4 - Prob. 12P
Ch. 9.4 - Prob. 13PCh. 9.5 - What product will be obtained from the reaction of...Ch. 9.5 - Prob. 15PCh. 9.6 - a. What is each ethers systematic name? 1....Ch. 9.8 - Draw the structure of the following: a....Ch. 9.8 - Prob. 20PCh. 9.8 - Would you expect the reactivity of a five-membered...Ch. 9.9 - Explain why the two arene oxides in Problem 22...Ch. 9.9 - Which compound is more likely to be...Ch. 9.11 - The following three nitrogen mustards were studied...Ch. 9 - What are the common and systematic names of the...Ch. 9 - Prob. 28PCh. 9 - Prob. 29PCh. 9 - Prob. 30PCh. 9 - Prob. 31PCh. 9 - What is the major product obtained from the...Ch. 9 - Draw structures for the following: a....Ch. 9 - Prob. 34PCh. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Ethylene oxide reacts readily with HO.because of...Ch. 9 - Propose a mechanism for each of the following...Ch. 9 - Which of the following ethers would be obtained in...Ch. 9 - Show how each of the following syntheses could be...Ch. 9 - Prob. 42PCh. 9 - Prob. 43PCh. 9 - Prob. 44PCh. 9 - Propose a mechanism for each of the following...Ch. 9 - a. Propose a mechanism for the following reaction:...Ch. 9 - Three arene oxides can be obtained from...Ch. 9 - Prob. 48PCh. 9 - The following reaction takes place several times...Ch. 9 - Show how each of the following compounds could be...Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - What alkenes would you expect to be obtained from...Ch. 9 - Triethylenemelamine (TEM) is an antitumor agent....Ch. 9 - When a diol that has OH groups on adjacent carbons...Ch. 9 - What product is obtained when...Ch. 9 - Prob. 58P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Step 1: add a curved arrow. Select Draw Templates More / " C H Br 0 Br : :o: Erase H H H H Q2Q Step 2: Draw the intermediates and a curved arrow. Select Draw Templates More MacBook Air / " C H Br 0 9 Q Erase 2Qarrow_forwardO Macmillan Learning Question 23 of 26 > Stacked Step 7: Check your work. Does your synthesis strategy give a substitution reaction with the expected regiochemistry and stereochemistry? Draw the expected product of the forward reaction. - - CN DMF MacBook Air Clearly show stereochemistry. Questionarrow_forwardNH2 1. CH3–MgCl 2. H3O+ ? As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will make a molecule with a new C - C bond as its major product: If this reaction will work, draw the major organic product or products you would expect in the drawing area below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and dash bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C - C bond, just check the box under the drawing area and leave it blank. Click and drag to start drawing a structure. This reaction will not make a product with a new C - C bond. Х ☐: Carrow_forward
- Predict the major products of this organic reaction. If there will be no major products, check the box under the drawing area instead. No reaction. : + Х è OH K Cr O 2 27 2 4' 2 Click and drag to start drawing a structure.arrow_forwardLaminar compounds are characterized by havinga) a high value of the internal surface of the solid.b) a high adsorption potential.arrow_forwardIntercalation compounds have their sheetsa) negatively charged.b) positively charged.arrow_forward
- Indicate whether the following two statements are correct or not:- Polythiazine, formed by N and S, does not conduct electricity- Carbon can have a specific surface area of 3000 m2/garrow_forwardIndicate whether the following two statements are correct or not:- The S8 heterocycle is the origin of a family of compounds- Most of the elements that give rise to stable heterocycles belong to group d.arrow_forwardcould someone draw curly arrow mechanism for this question pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning  Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License