
Concept explainers
(a)
Interpretation:
The alcohol in the given pair that undergoes an elimination reaction more rapidly when heated with
Concept introduction:
Dehydration reaction:
Removal of water molecule from the alcohol in the presence of strong acid like sulfuric acid is known as dehydration reaction.
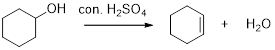
Carbocation: It is carbon ion that bears a positive charge on it.
The increasing stability order of carbocation is as follows,
Primary carbocation < secondary carbocation < tertiary carbocation
(b)
Interpretation:
The alcohol in the given pair that undergoes an elimination reaction more rapidly when heated with
Concept introduction:
Dehydration reaction:
Removal of water molecule from the alcohol in the presence of strong acid like sulfuric acid is known as dehydration reaction.
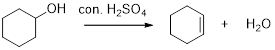
Carbocation: It is carbon ion that bears a positive charge on it.
The increasing stability order of carbocation is as follows,
Primary carbocation < secondary carbocation < tertiary carbocation
(c)
Interpretation:
The alcohol in the given pair that undergoes an elimination reaction more rapidly when heated with
Concept introduction:
Dehydration reaction:
Removal of water molecule from the alcohol in the presence of strong acid like sulfuric acid is known as dehydration reaction.
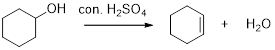
Carbocation: It is carbon ion that bears a positive charge on it.
The increasing stability order of carbocation is as follows,
Primary carbocation < secondary carbocation < tertiary carbocation
(d)
Interpretation:
The alcohol in the given pair that undergoes an elimination reaction more rapidly when heated with
Concept introduction:
Dehydration reaction:
Removal of water molecule from the alcohol in the presence of strong acid like sulfuric acid is known as dehydration reaction.
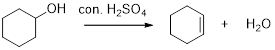
Carbocation: It is carbon ion that bears a positive charge on it.
The increasing stability order of carbocation is as follows,
Primary carbocation < secondary carbocation < tertiary carbocation
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Essential Organic Chemistry, Global Edition
- 4. Experimental Procedure. a. How many (total) data plots are to be completed for this experiment? Account for each. b. What information is to be extracted from each data plot?arrow_forwardProvide the IUPAC name of the following molecule. Don't forget to include the proper stereochemistry where appropriate.arrow_forward3. 2. 1. On the graph below, plot the volume of rain in milliliters versus its height in centimeters for the 400 mL beaker. Draw a straight line through the points and label it "400 mL beaker." Volume (mL) 400 350 300 250 200 150 750 mL Florence Volume Versus Height of Water 400 mL beaker 100 50 0 0 2 3 4 5 Height (cm) 6 7 8 9 10 Explain why the data points for the beaker lie roughly on a straight line. What kind of relationship is this? How do you know? (see page 276 text) the design of the beaker is a uniform cylinder the volume of liquid increases evenly with its height resulting in a linear relationship. What volume would you predict for 10.0 cm of water? Explain how you arrived at your answer. Use the data table and the graph to assist you in answering the question. 4. Plot the volume of rain in milliliters versus its height in centimeters for the 250 mL Florence flask on the same graph. Draw a best-fit curve through the points and label it "250 mL Florence flask." oke camearrow_forward
- Show work. Don't give Ai generated solutionarrow_forwardIn the video, we looked at the absorbance of a certain substance and how it varies depending on what wavelength of light we are looking at. Below is a similar scan of a different substance. What color BEST describes how this substance will appear? Absorbance (AU) Violet Blue Green Orange 1.2 1.0- 0.8- 0.6- 0.4- 0.2 0.0 450 500 550 600 650 700 Wavelength (nm) violet indigo blue green yellow orange red Red O Cannot tell from this information In the above graph, what causes -450 nm wavelength of light to have a higher absorbance than light with a -550 nm wavelength? Check all that are true. The distance the light travels is different The different data points are for different substances The concentration is different at different times in the experiment Epsilon (molar absortivity) is different at different wavelengthsarrow_forward5. a. Data were collected for Trial 1 to determine the molar mass of a nonvolatile solid solute when dissolved in cyclo- hexane. Complete the table for the analysis (See Report Sheet). Record calculated values with the correct number of significant figures. B. Freezing Point of Cyclohexane plus Calculation Zone Unknown Solute 2. Mass of cyclohexane (g) 10.14 Part C.4 3. Mass of added solute (g) 0.255 C. Calculations 1. k; for cyclohexane (°C⚫ kg/mol) 20.0 2. Freezing point change, AT, (°C) 3.04 Part C.6 3. Mass of cyclohexane in solution (kg) 4. Moles of solute, total (mol) Show calculation. 5. Mass of solute in solution, total (g) 6. Molar mass of solute (g/mol) Show calculation.arrow_forward
- Draw and name the R groups of all 20 amino acids.arrow_forward3. Two solutions are prepared using the same solute: Solution A: 0.14 g of the solute dissolves in 15.4 g of t-butanol Solution B: 0.17 g of the solute dissolves in 12.7 g of cyclohexane Which solution has the greatest freezing point change? Show calculations and explain.arrow_forward2. Give the ground state electron configuration (e.g., 02s² σ*2s² П 2p²) for these molecules and deduce its bond order. Ground State Configuration Bond Order H2+ 02- N2arrow_forward
- 1. This experiment is more about understanding the colligative properties of a solution rather than the determination of the molar mass of a solid. a. Define colligative properties. b. Which of the following solutes has the greatest effect on the colligative properties for a given mass of pure water? Explain. (i) 0.01 mol of CaCl2 (ii) 0.01 mol of KNO3 (iii) 0.01 mol of CO(NH2)2 (an electrolyte) (an electrolyte) (a nonelectrolyte)arrow_forward5. b. For Trials 2 and 3, the molar mass of the solute was 151 g/mol and 143 g/mol respectively. a. What is the average molar mass of the solute ? b. What are the standard deviation and the relative standard deviation (%RSD) for the molar mass of the solute ?arrow_forwardShow work. Don't give Ai generated solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





