
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 56P
When a
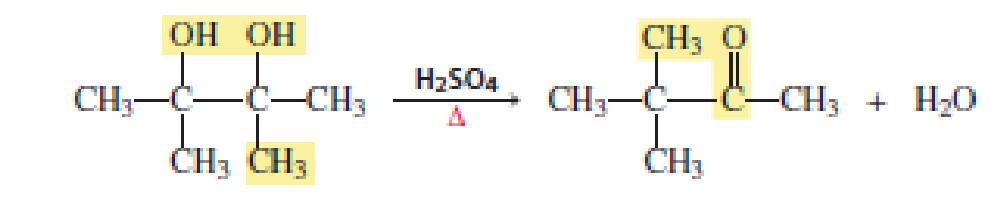
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1) Draw the control charts for the following data and
interpret the result and also develop control limts for
future use. 24 samples are taken each with a subgroup
size of 3.
Don't Use the standard excel template and analyze.
1) Draw the control charts for the following data and interpret the result and also develop
control limts for future use. 24 samples are taken each with a subgroup size of 3.
Problem to be solved both as an assignment and laboratory.
Subgroup
X₁
X2
X3
1
7
8
10
2
9
9
14
3
15
16
10
4
14
13
15
5
12
11
10
6
10
11
9
I
7
10
9
9
8
15
17
13
9
10
7
8
10
9
8
9
11
8
8
10
12
17
13
10
13
10
12
11
14
9
9
10
15
10
8
8
16
11
10
9
17
10
10
8
18
8
9
7
19
9
8
9
22222
10
10
11
9
10
9
11
9
10
12
12
11
14
2012 4
How much of each solution should be
used to prepare 1L of a buffer
solution with a pH of 9.45 using 3M
Na2CO3 and 0.2M HCI? Given: Ka
1 = 4.3 × 10-7, Ka2 = 4.69 × 10-11
Chapter 9 Solutions
Essential Organic Chemistry, Global Edition
Ch. 9.1 - Draw the structures of straight-chain alcohols...Ch. 9.1 - Prob. 2PCh. 9.1 - Prob. 3PCh. 9.2 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 9.2 - Prob. 5PCh. 9.2 - The observed relative reactivities of primary,...Ch. 9.4 - Which of the following alcohols would dehydrate...Ch. 9.4 - Prob. 10PCh. 9.4 - Prob. 11PCh. 9.4 - Prob. 12P
Ch. 9.4 - Prob. 13PCh. 9.5 - What product will be obtained from the reaction of...Ch. 9.5 - Prob. 15PCh. 9.6 - a. What is each ethers systematic name? 1....Ch. 9.8 - Draw the structure of the following: a....Ch. 9.8 - Prob. 20PCh. 9.8 - Would you expect the reactivity of a five-membered...Ch. 9.9 - Explain why the two arene oxides in Problem 22...Ch. 9.9 - Which compound is more likely to be...Ch. 9.11 - The following three nitrogen mustards were studied...Ch. 9 - What are the common and systematic names of the...Ch. 9 - Prob. 28PCh. 9 - Prob. 29PCh. 9 - Prob. 30PCh. 9 - Prob. 31PCh. 9 - What is the major product obtained from the...Ch. 9 - Draw structures for the following: a....Ch. 9 - Prob. 34PCh. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Ethylene oxide reacts readily with HO.because of...Ch. 9 - Propose a mechanism for each of the following...Ch. 9 - Which of the following ethers would be obtained in...Ch. 9 - Show how each of the following syntheses could be...Ch. 9 - Prob. 42PCh. 9 - Prob. 43PCh. 9 - Prob. 44PCh. 9 - Propose a mechanism for each of the following...Ch. 9 - a. Propose a mechanism for the following reaction:...Ch. 9 - Three arene oxides can be obtained from...Ch. 9 - Prob. 48PCh. 9 - The following reaction takes place several times...Ch. 9 - Show how each of the following compounds could be...Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - What alkenes would you expect to be obtained from...Ch. 9 - Triethylenemelamine (TEM) is an antitumor agent....Ch. 9 - When a diol that has OH groups on adjacent carbons...Ch. 9 - What product is obtained when...Ch. 9 - Prob. 58P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Add substituents to draw the conformer below (sighting down the indicated bond), then rotate the back carbon to provide the anti staggered conformer. + H3C H Ph H Problem 25 of 30 Drawing Atoms, Bonds and Rings Charges Tap a node to see suggestions H H H Undo Rasat Remove Done Finish update Rotate Submitarrow_forwardwhat temperature does a 50% (mole fraction) of ammonia/water liquid mixture boil at 1 atmarrow_forward1) Suppose 0.1 kg ice at 0°C (273K) is in 0.5kg water at 20°C (293K). What is the change in entropy of the ice as it melts at 0°? To produce the original "water gas" mixture, carbon (in a combustible form known as coke) is reacted with steam: 131.4 kJ + H20(g) + C(s) → CO(g) + H2(g) From this information and the equations in the previous problem, calculate the enthalpy for the combustion or carbon to form carbon dioxide. kindly show me how to solve both parts of the same long problem. Thanksarrow_forward
- we were assigned to dilute 900ppm in to 18ppm by using only 250ml vol flask. firstly we did calc and convert 900ppm to 0.9 ppm to dilute in 1 liter. to begin the experiment we took 0,225g of kmno4 and dissolved in to 250 vol flask. then further we took 10 ml sample sol and dissolved in to 100 ml vol flask and put it in to a spectrometer and got value of 0.145A . upon further calc we got v2 as 50ml . need to find DF, % error (expval and accptVal), molarity, molality. please write the whole report. thank you The format, tables, introduction, procedure and observation, result, calculations, discussion and conclusionarrow_forwardQ5. Predict the organic product(s) for the following transformations. If no reaction will take place (or the reaction is not synthetically useful), write "N.R.". Determine what type of transition state is present for each reaction (think Hammond Postulate). I Br₂ CH3 F2, light CH3 Heat CH3 F₂ Heat Br2, light 12, light CH3 Cl2, light Noarrow_forwardNonearrow_forward
- In the phase diagram of steel (two components Fe and C), region A is the gamma austenite solid and region B contains the gamma solid and liquid. Indicate the degrees of freedom that the fields A and B have,arrow_forwardFor a condensed binary system in equilibrium at constant pressure, indicate the maximum number of phases that can exist.arrow_forwardPart V. Label ad match the carbons in compounds Jane and Diane w/ the corresponding peak no. in the Spectra (Note: use the given peak no. To label the carbons, other peak no are intentionally omitted) 7 4 2 -0.13 -0.12 -0.11 -0.10 -0.08 8 CI Jane 1 -0.09 5 210 200 190 180 170 160 150 140 130 120 110 100 -8 90 f1 (ppm) 11 8 172.4 172.0 f1 (ppr HO CI NH Diane 7 3 11 80 80 -80 -R 70 60 60 2 5 -8 50 40 8. 170 160 150 140 130 120 110 100 90 -0 80 70 20 f1 (ppm) 15 30 -20 20 -60 60 -0.07 -0.06 -0.05 -0.04 -0.03 -0.02 -0.01 -0.00 -0.01 10 -0.17 16 15 56 16 -0.16 -0.15 -0.14 -0.13 -0.12 -0.11 -0.10 -0.09 -0.08 -0.07 -0.06 -0.05 -0.04 17.8 17.6 17.4 17.2 17.0 f1 (ppm) -0.03 -0.02 550 106 40 30 20 20 -0.01 -0.00 F-0.01 10 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY