
General, Organic, and Biological Chemistry
7th Edition
ISBN: 9781285853918
Author: H. Stephen Stoker
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 9, Problem 9.83EP
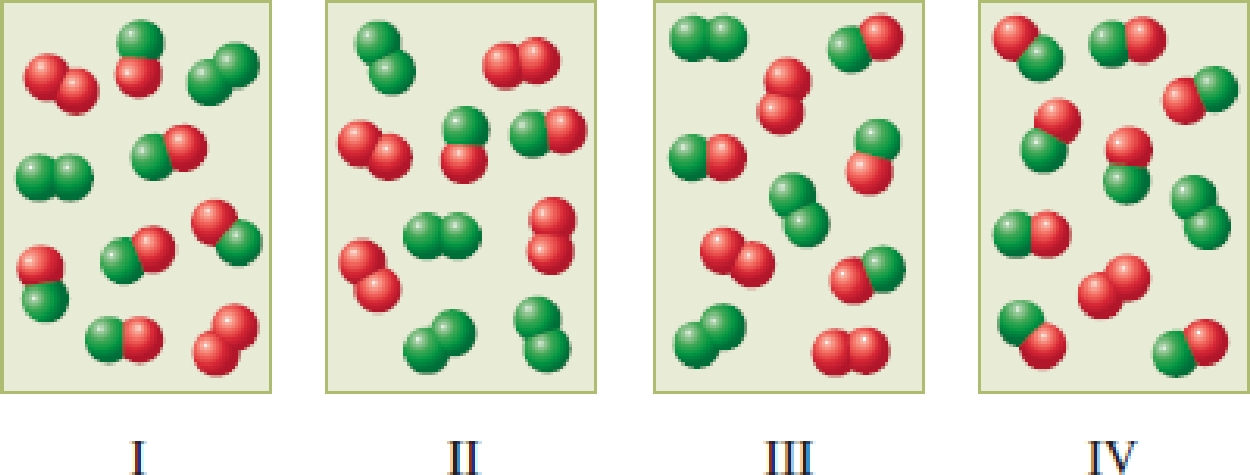
The following four diagrams represent gaseous reaction mixtures for the
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
So the thing is im trying to memorize VESPR Shapes in order to be able to solve problems like so, and I need help with making circles like the second image that's in blue or using an x and y axis plane in order to memorize these and be able to solve those type of problems. Especially like the ones given in the top / first image. (180 , 120 , 109.5) Can you help me with this.
Don't used hand raiting and don't used Ai solution
2. (15 points) Draw an appropriate mechanism for the following reaction.
H
N.
H*
+ H₂O
Chapter 9 Solutions
General, Organic, and Biological Chemistry
Ch. 9.1 - Prob. 1QQCh. 9.1 - Prob. 2QQCh. 9.1 - Prob. 3QQCh. 9.2 - The proper assignment of oxidation numbers to the...Ch. 9.2 - The proper assignment of oxidation numbers to the...Ch. 9.2 - Prob. 3QQCh. 9.3 - Prob. 1QQCh. 9.3 - Prob. 2QQCh. 9.3 - Prob. 3QQCh. 9.3 - Prob. 4QQ
Ch. 9.3 - Prob. 5QQCh. 9.4 - Prob. 1QQCh. 9.4 - Prob. 2QQCh. 9.4 - Prob. 3QQCh. 9.5 - Prob. 1QQCh. 9.5 - Prob. 2QQCh. 9.5 - For endothermic chemical reactions the energy...Ch. 9.6 - Prob. 1QQCh. 9.6 - Prob. 2QQCh. 9.6 - Prob. 3QQCh. 9.7 - Prob. 1QQCh. 9.7 - Prob. 2QQCh. 9.7 - Prob. 3QQCh. 9.8 - Which of the following is the correct equilibrium...Ch. 9.8 - Prob. 2QQCh. 9.8 - Prob. 3QQCh. 9.9 - Prob. 1QQCh. 9.9 - Prob. 2QQCh. 9.9 - Prob. 3QQCh. 9.9 - Prob. 4QQCh. 9 - What is the general chemical equation for each of...Ch. 9 - What is the general chemical equation for each of...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Write the chemical formulas for the products...Ch. 9 - Write the chemical formulas for the products...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - Indicate to which of the following types of...Ch. 9 - Indicate to which of the following types of...Ch. 9 - What is the oxidation number of S in each of the...Ch. 9 - Prob. 9.12EPCh. 9 - Determine the oxidation number of the indicated...Ch. 9 - Determine the oxidation number of the indicated...Ch. 9 - Prob. 9.15EPCh. 9 - Prob. 9.16EPCh. 9 - What is the oxidation number of each element...Ch. 9 - What is the oxidation number of each element...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Classify each of the following reactions as a...Ch. 9 - Classify each of the following reactions as (1) a...Ch. 9 - Prob. 9.22EPCh. 9 - Classify each of the following reactions using one...Ch. 9 - Classify each of the following reactions using one...Ch. 9 - Prob. 9.25EPCh. 9 - In each of the following changes is the reactant...Ch. 9 - Identify which substance is oxidized and which...Ch. 9 - Identify which substance is oxidized and which...Ch. 9 - Prob. 9.29EPCh. 9 - Prob. 9.30EPCh. 9 - Indicate whether each of the following substances...Ch. 9 - Indicate whether each of the following substances...Ch. 9 - Prob. 9.33EPCh. 9 - Prob. 9.34EPCh. 9 - What are the three central concepts associated...Ch. 9 - Why are most chemical reactions carried out either...Ch. 9 - What two factors determine whether a collision...Ch. 9 - What happens to the reactants in an ineffective...Ch. 9 - Which of the following reactions are endothermic,...Ch. 9 - Prob. 9.40EPCh. 9 - Should heat be added as a reactant or as a product...Ch. 9 - Should heat be added as a reactant or as a product...Ch. 9 - Prob. 9.43EPCh. 9 - Indicate whether each of the following is a...Ch. 9 - Sketch an energy diagram graph representing an...Ch. 9 - Sketch an energy diagram graph representing an...Ch. 9 - Using collision theory, indicate why each of the...Ch. 9 - Using collision theory, indicate why each of the...Ch. 9 - Substances burn more rapidly in pure oxygen than...Ch. 9 - Milk will sour in a couple of days when left at...Ch. 9 - Will each of the changes listed increase or...Ch. 9 - Will each of the changes listed increase or...Ch. 9 - For each of the changes listed will the rate of...Ch. 9 - For each of the changes listed will the rate of...Ch. 9 - Prob. 9.55EPCh. 9 - Draw an energy diagram graph for an endothermic...Ch. 9 - The characteristics of four reactions, each of...Ch. 9 - The characteristics of four reactions, each of...Ch. 9 - What condition must be met in order for a system...Ch. 9 - What relationship exists between the rates of the...Ch. 9 - What does the term reversible reaction mean?Ch. 9 - What does the notation denote when it is used in...Ch. 9 - Consider the following equilibrium system....Ch. 9 - Consider the following equilibrium system....Ch. 9 - Prob. 9.65EPCh. 9 - Sketch a graph showing how the rates of the...Ch. 9 - The following series of diagrams represent the...Ch. 9 - The following series of diagrams represent the...Ch. 9 - For the reaction A2 + 2B 2AB, diagram I depicts...Ch. 9 - For the reaction A2 + B2 2AB, diagram I depicts...Ch. 9 - Write equilibrium constant expressions for the...Ch. 9 - Write equilibrium constant expressions for the...Ch. 9 - Write equilibrium constant expressions for the...Ch. 9 - Prob. 9.74EPCh. 9 - Calculate the value of the equilibrium constant...Ch. 9 - Calculate the value of the equilibrium constant...Ch. 9 - Prob. 9.77EPCh. 9 - Use the given Keq value and the terminology in...Ch. 9 - Write a balanced chemical equation for a totally...Ch. 9 - Write a balanced chemical equation for a totally...Ch. 9 - The following four diagrams represent gaseous...Ch. 9 - Based on the diagrams, chemical reaction, and...Ch. 9 - The following four diagrams represent gaseous...Ch. 9 - Based on the diagrams, chemical reaction, and...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - Indicate whether or not each of the following...Ch. 9 - For the generalized chemical reaction...Ch. 9 - For the generalized chemical reaction...Ch. 9 - Prob. 9.89EPCh. 9 - For the reaction C6H6(g)+3H2(g)C6H12(g)+heat...Ch. 9 - Consider the following chemical system at...Ch. 9 - Prob. 9.92EPCh. 9 - The following two diagrams represent the...Ch. 9 - The following two diagrams represent the...Ch. 9 - Indicate whether or not product formation...Ch. 9 - Prob. 9.96EPCh. 9 - Prob. 9.97EPCh. 9 - Indicate whether or not product formation...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a tripeptide of your choosing at pH 7. Have the N-terminus on the left and the C-terminus on the right. Then: Draw a triangle around the α-carbons. Draw a box around the R-groups. Circle the atoms capable of hydrogen bonding. Highlight the atoms involved in the formation of the peptide bonds. What type of structure have you drawn? (primary, secondary, tertiary or quaternary protein structure). make sure its a tripeptidearrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used Ai solution and don't used hand raitingarrow_forward> Organic Functional Groups Naming and drawing alkyl halides structure CI Br CI CI Explanation Check 2 name 1-chloro-2,4,9-trimethylnonane CI 2-iodo-2,3-dimethylbutane FEB 19 € E M tv MacBook Airarrow_forwardCan you please explain to me this problem im very confused and lost. Help me step by step and in detail im soo lost.arrow_forward
- 2) There are many forms of cancer, all of which involve abnormal cell growth. The growth and production of cells, called cell proliferation, is known to involve an enzyme called protein farnesyltransferase (PFTase). It is thought that inhibitors pf PFTase may be useful as anticancer drugs. The following molecule showed moderate activity as a potential PFTase inhibitor. Draw all stereoisomers of this compound. HO OHarrow_forwardConsidering rotation around the bond highlighted in red, draw the Newman projection for the most stable and least stable conformations when viewed down the red bond in the direction of the arrow. Part 1 of 2 H₁₂C H H Draw the Newman projection for the most stable conformation. Select a template to begin. Part 2 of 2 Draw the Newman projection for the least stable conformation. G 心arrow_forwardpersonality of each of them in terms of nucleophile vs. electrophile (some can be considered acids/bases but we are not looking at that here). Note you may have to use your growing intuition to figure out the personality of one of the molecules below but I believe in you! Rationalize it out based on what we have called strong versus weak electrophiles in past mechanisms. Consider using the memes below to help guide your understanding! A OH O B CH3 C Molecule A: [Select] Molecule B: [Select] Molecule C: [Select] Molecule D: [Select] > H D OHarrow_forward
- 4) Which oxygen atom in the structure below is most basic / nucleophilic? Please explain by discussing the electron density around each oxygen atom. Show at least three resonance structures for the compound. оогоarrow_forwardCan you show me this problem. Turn them into lewis dot structures for me please and then answer the question because I cant seem to comprehend it/ The diagrams on the picture look too small I guess.arrow_forwardThe fire releases 2.80 x 107 Joules of heat energy for each liter of oil burned. The water starts out at 24.5 °C, raising the water's temperature up to 100 °C, and then raises the temperature of the resulting steam up to 325 °C. How many liters of water will be needed to absorb the heat from the fire in this way, for each 1.0 liter of crude oil burned? 4186 J/(kg°C) = heat of water 2020 J/(kg°C) = heat of steam 2,256,000 (i.e. 2.256 x 106) J/kg = latent heat of vaporization for water (at the boiling point of 100 °C).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY