
Concept explainers
(a)
Interpretation: The indicated compound should be named.
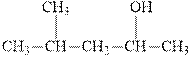
Concept introduction: In accordance with
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain. The longest chain must contain the alcohol
The chain carbons are numbered so as to provide least numeral position towards the end in vicinity to
For
(b)
Interpretation:The indicated compound should be named.
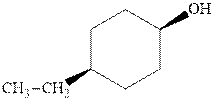
Concept introduction:In accordance with IUPAC systematic nomenclature od alcohols can be derived from the parent alkane with “e” dropped and replaced by “ol” suffix.
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain. The longest chain must contain the alcohol functional group.
The chain carbons are numbered so as to provide least numeral position towards the end in vicinity to
For alkanes side chains the positions of side chains or alkyl substituents are indicated by lowest numeral places before prefix.
(c)
Interpretation: The indicated compound should be named.
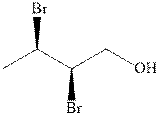
Concept introduction:In accordance with IUPAC systematic nomenclature od alcohols can be derived from the parent alkane with “e” dropped and replaced by “ol” suffix.
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain. The longest chain must contain the alcohol functional group.
The chain carbons are numbered so as to provide least numeral position towards the end in vicinity to
For alkanes side chains the positions of side chains or alkyl substituents are indicated by lowest numeral places before prefix.
In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order on the basis of
The Fischer projection is written along with the priorities assigned and groups are interchanged between adjacent places so as to obtain lowest priority group at the bottom or lowest priority.
If the groups now are arranged are read from highest towards least in clockwise fashion the R is assigned to the stereocenter, if the rotation is anticlockwise the S is assigned at the configuration.
(d)
Interpretation: The indicated compound should be named.
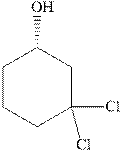
Concept introduction:In accordance with IUPAC systematic nomenclature od alcohols can be derived from the parent alkane with “e” dropped and replaced by “ol” suffix.
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain. The longest chain must contain the alcohol functional group.
The chain carbons are numbered so as to provide least numeral position towards the end in vicinity to
For alkanes side chains the positions of side chains or alkyl substituents are indicated by lowest numeral places before prefix.
In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order on the basis of atomic number. The one with highest atomic number gest highest priority and is designated as a and so on.
The Fischer projection is written along with the priorities assigned and groups are interchanged between adjacent places so as to obtain lowest priority group at the bottom or lowest priority.
If the groups now are arranged are read from highest towards least in clockwise fashion the R is assigned to the stereocenter, if the rotation is anticlockwise the S is assigned at the configuration.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
ORGANIC CHEMISTRY (LL)-PACKAGE
- A partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forwardProblem 38 can u explain and solve thanks april 24arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





