
Concept explainers
(a)
Interpretation: Thepossible synthetic route for synthesis of menthol from first
Concept introduction: The carbonyl bond is polar with the partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
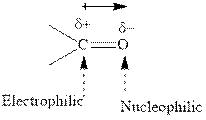
Grignard reagents are
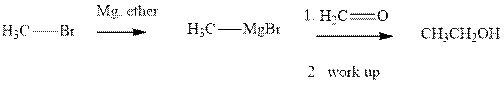
Methyl bromide reacts with magnesium ether to give Grignard reagent. This Grignard methyl magnesium bromide on treatment with formaldehyde gives corresponding alcohol. The mechanistic pathway for the latter reaction is as follows:

The polar carbonyl bond breaks and the polar Grignard reagent attack at electron-deficient carbon. Finally with the hydrolysis and work up the alcohol is formed.
(b)
Interpretation: The possible synthetic route for synthesis of ethanol from first aldehyde and second a ketone should be suggested.
Concept introduction: The carbonyl bond is polar with the partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
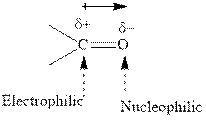
Grignard reagents are alkyl magnesium halides obtained from treatment of haloalkane with magnesium in presence of dried ether conditions. These reagents are useful precursors for quick synthesis of variety of organic compounds For example, the reaction of Grignard reagent with aldehyde or ketone generates alcohol as illustrated below.
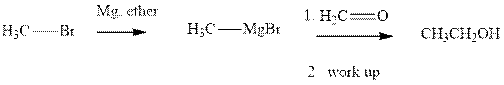
Methyl bromide reacts with magnesium ether to give Grignard reagent. This Grignard methyl magnesium bromide on treatment with formaldehyde gives corresponding alcohol. The mechanistic pathway for the latter reaction is as follows:

The polar carbonyl bond breaks and the polar Grignard reagent attack at electron-deficient carbon. Finally with the hydrolysis and work up the alcohol is formed.
(c)
Interpretation: The possible synthetic route for synthesis of
Concept introduction: The carbonyl bond is polar with the partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
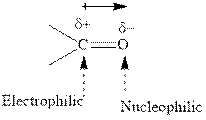
Grignard reagents are alkyl magnesium halides obtained from treatment of haloalkane with magnesium in presence of dried ether conditions. These reagents are useful precursors for quick synthesis of variety of organic compounds For example, the reaction of Grignard reagent with aldehyde or ketone generates alcohol as illustrated below.
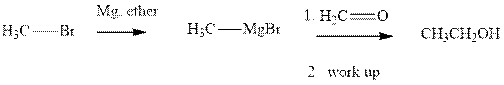
Methyl bromide reacts with magnesium ether to give Grignard reagent. This Grignard methyl magnesium bromide on treatment with formaldehyde gives corresponding alcohol. The mechanistic pathway for the latter reaction is as follows:

The polar carbonyl bond breaks and the polar Grignard reagent attack at electron-deficient carbon. Finally with the hydrolysis and work up the alcohol is formed.
(d)
Interpretation: The possible synthetic route for synthesis of
Concept introduction: The carbonyl bond is polar with the partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
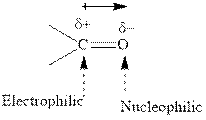
Grignard reagents are alkyl magnesium halides obtained from treatment of haloalkane with magnesium in presence of dried ether conditions. These reagents are useful precursors for quick synthesis of variety of organic compounds For example, the reaction of Grignard reagent with aldehyde or ketone generates alcohol as illustrated below.
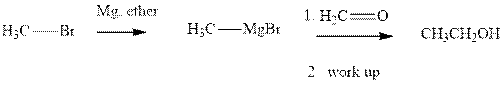
Methyl bromide reacts with magnesium ether to give Grignard reagent. This Grignard methyl magnesium bromide on treatment with formaldehyde gives corresponding alcohol. The mechanistic pathway for the latter reaction is as follows:

The polar carbonyl bond breaks and the polar Grignard reagent attack at electron-deficient carbon. Finally with the hydrolysis and work up the alcohol is formed.
(e)
Interpretation: The possible synthetic route for synthesis of
Concept introduction: The carbonyl bond is polar with the partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
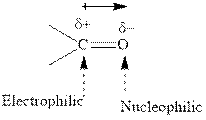
Grignard reagents are alkyl magnesium halides obtained from treatment of haloalkane with magnesium in presence of dried ether conditions. These reagents are useful precursors for quick synthesis of variety of organic compounds For example, the reaction of Grignard reagent with aldehyde or ketone generates alcohol as illustrated below.
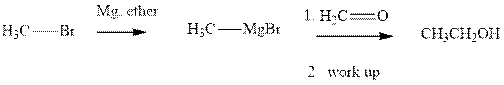
Methyl bromide reacts with magnesium ether to give Grignard reagent. This Grignard methyl magnesium bromide on treatment with formaldehyde gives corresponding alcohol. The mechanistic pathway for the latter reaction is as follows:

The polar carbonyl bond breaks and the polar Grignard reagent attack at electron-deficient carbon. Finally with the hydrolysis and work up the alcohol is formed.
(f)
Interpretation: The possible synthetic route for synthesis of
Concept introduction: The carbonyl bond is polar with the partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
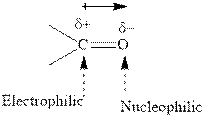
Grignard reagents are alkyl magnesium halides obtained from treatment of haloalkane with magnesium in presence of dried ether conditions. These reagents are useful precursors for quick synthesis of variety of organic compounds For example, the reaction of Grignard reagent with aldehyde or ketone generates alcohol as illustrated below.
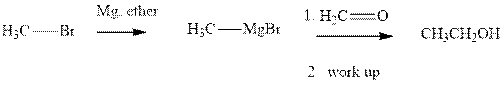
Methyl bromide reacts with magnesium ether to give Grignard reagent. This Grignard methyl magnesium bromide on treatment with formaldehyde gives corresponding alcohol. The mechanistic pathway for the latter reaction is as follows:

The polar carbonyl bond breaks and the polar Grignard reagent attack at electron-deficient carbon. Finally with the hydrolysis and work up the alcohol is formed.
(g)
Interpretation: The possible synthetic route for synthesis of
Concept introduction: The carbonyl bond is polar with the partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
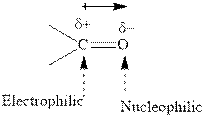
Grignard reagents are alkyl magnesium halides obtained from treatment of haloalkane with magnesium in presence of dried ether conditions. These reagents are useful precursors for quick synthesis of variety of organic compounds For example, the reaction of Grignard reagent with aldehyde or ketone generates alcohol as illustrated below.
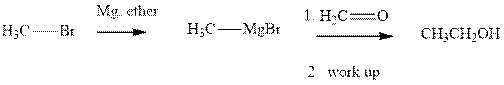
Methyl bromide reacts with magnesium ether to give Grignard reagent. This Grignard methyl magnesium bromide on treatment with formaldehyde gives corresponding alcohol. The mechanistic pathway for the latter reaction is as follows:

The polar carbonyl bond breaks and the polar Grignard reagent attack at electron-deficient carbon. Finally with the hydrolysis and work up the alcohol is formed.
Trending nowThis is a popular solution!

Chapter 8 Solutions
ORGANIC CHEMISTRY (LL)-PACKAGE
- What is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forward
- Predict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forwardPredict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forward
- Predict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forwardYou are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forward
- Predict the products of this organic reaction: CH3 O CH3-CH-C-O-CH2-CH2-CH3 + H₂OH+ Η ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward€ CH3-CH-C-O-CH2-CH2-CH3 + NaOH A? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Predict the products of this organic reaction: CH3 O Click anywhere to draw the first atom of your structure. No reaction ✓ Garrow_forwardA molecule can have a temporary or permanent depending on the structure and the way the electrons can move. True Falsearrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


