
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 82P
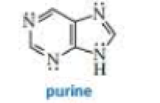
Purine is a heterocyclic compound with four nitrogen atoms.
- a. Which nitrogen is most apt to be protonated?
- b. Which nitrogen is least apt to be protonated?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Estimation of ash in food
Questions:
Q1: What does the word ash
refer to?
Q2: Mention the types of ash in
food
Q3: Mention the benefit of using
a glass dryer
Draw structures corresponding to the names given
a. m-fluoronitrobenzene
b. p-bromoaniline
c. o-chlorophenol
d. 3,5-dimethylbenzoic acid
Illustrate the reaction mechanism the following reaction
Chapter 8 Solutions
EBK ORGANIC CHEMISTRY
Ch. 8.1 - Prob. 1PCh. 8.1 - Prob. 2PCh. 8.4 - Prob. 3PCh. 8.5 - Prob. 4PCh. 8.5 - Prob. 6PCh. 8.6 - a. Predict the relative bond lengths of the three...Ch. 8.6 - Prob. 8PCh. 8.6 - Prob. 9PCh. 8.6 - Prob. 10PCh. 8.7 - Prob. 11P
Ch. 8.7 - Prob. 12PCh. 8.7 - Prob. 13PCh. 8.8 - Prob. 14PCh. 8.8 - Prob. 15PCh. 8.8 - Prob. 16PCh. 8.9 - Which member of each pair is the stronger acid?Ch. 8.9 - Which member of each pair is the stronger base? a....Ch. 8.9 - Rank the following compounds from strongest acid...Ch. 8.10 - Prob. 20PCh. 8.10 - Which acid in each of the following pairs is...Ch. 8.10 - Prob. 23PCh. 8.11 - Prob. 24PCh. 8.11 - Prob. 26PCh. 8.12 - Prob. 27PCh. 8.12 - Prob. 28PCh. 8.12 - Prob. 29PCh. 8.12 - Prob. 30PCh. 8.12 - Prob. 31PCh. 8.12 - Prob. 32PCh. 8.13 - Prob. 33PCh. 8.13 - Prob. 34PCh. 8.13 - Prob. 35PCh. 8.13 - What are the major 1,2- and 1,4-addition products...Ch. 8.13 - Prob. 38PCh. 8.14 - Prob. 39PCh. 8.14 - Prob. 40PCh. 8.14 - Prob. 41PCh. 8.14 - Prob. 42PCh. 8.14 - Prob. 43PCh. 8.14 - Prob. 44PCh. 8.14 - Prob. 46PCh. 8.15 - Prob. 47PCh. 8.17 - Prob. 48PCh. 8.17 - Prob. 49PCh. 8.18 - Prob. 50PCh. 8.18 - Prob. 52PCh. 8.18 - Prob. 53PCh. 8.18 - Prob. 54PCh. 8.19 - Prob. 55PCh. 8.20 - Prob. 56PCh. 8.20 - What orbitals contain the electrons represented as...Ch. 8.20 - Prob. 59PCh. 8.20 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - Prob. 64PCh. 8 - Prob. 65PCh. 8 - Prob. 66PCh. 8 - Prob. 67PCh. 8 - Prob. 68PCh. 8 - Prob. 69PCh. 8 - Prob. 70PCh. 8 - Prob. 71PCh. 8 - Prob. 72PCh. 8 - Prob. 73PCh. 8 - Which compound is the strongest base?Ch. 8 - Prob. 75PCh. 8 - Prob. 76PCh. 8 - a. The A ring (Section 3.16) of cortisone (a...Ch. 8 - Prob. 78PCh. 8 - Prob. 79PCh. 8 - Prob. 80PCh. 8 - Prob. 81PCh. 8 - Purine is a heterocyclic compound with four...Ch. 8 - Prob. 83PCh. 8 - Why is the delocalization energy of pyrrole (21...Ch. 8 - Prob. 85PCh. 8 - Prob. 86PCh. 8 - Prob. 87PCh. 8 - A student obtained two products from the reaction...Ch. 8 - Prob. 89PCh. 8 - a. How could each of the following compounds be...Ch. 8 - Draw the products obtained from the reaction of...Ch. 8 - How would the following substituents affect the...Ch. 8 - Prob. 93PCh. 8 - The acid dissociation constant (Ka) for loss of a...Ch. 8 - Protonated cyclohexylamine has a Ka = 1 1011...Ch. 8 - Draw the product or products that would be...Ch. 8 - Prob. 97PCh. 8 - Prob. 98PCh. 8 - Prob. 99PCh. 8 - Prob. 100PCh. 8 - Prob. 101PCh. 8 - a. Propose n mechanism for the following reaction:...Ch. 8 - Prob. 103PCh. 8 - As many as 18 different Diels-Alder products can...Ch. 8 - Prob. 105PCh. 8 - Prob. 106PCh. 8 - Prob. 107PCh. 8 - Prob. 108PCh. 8 - The experiment shown next and discussed in Section...Ch. 8 - Prob. 110PCh. 8 - Prob. 111PCh. 8 - Prob. 112PCh. 8 - Prob. 1PCh. 8 - Prob. 2PCh. 8 - Prob. 3PCh. 8 - Prob. 4PCh. 8 - Prob. 5PCh. 8 - Prob. 6PCh. 8 - Prob. 7PCh. 8 - Prob. 8PCh. 8 - Prob. 9PCh. 8 - Prob. 10PCh. 8 - Prob. 11PCh. 8 - Prob. 12P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propose a synthesis for the following compound using benzene or toluene and any other reagents necessary. Show all major intermediate compounds that would probably be isolated during the course of your synthesis. on. Harrow_forwardProvide correct IUPAC names for each of the following compounds. NOT a. b. C. 2003 H,N- CH3 NH2 CHarrow_forward. Consider the reaction below to answer the following questions. OH 1. NaH 2. CH3I, ether O-CH3 A. Write the complete stepwise mechanism for the reaction. Show all intermediate structures and all electron flow with arrows. B. Mechanistically, the Williamson ether synthesis outlined above is: ن نخنه a. an El process b. an SN1 process C. an E2 process d. an SN2 process C. Alternatively, cyclopentyl methyl ether may be synthesized from cyclopentene. synthesis of cyclopentyl methyl ether from cyclopentene. Outline aarrow_forward
- Q2. A good synthesis of (CH3)3C- would be: A) B) CSI3 0 CH3CC1 (CH3) 3CC1 Benzene AlCl3 AlCl3 (CH3)3CC1 CH3CC1 Benzene C) AlCl3 0 AlCl3 CH3CC1 (CH3) 2C-CH2 Bonzone AlCl3 HF D) More than one of these E) None of thesearrow_forwardDon't used hand raiting and correct answer and don't used Ai solutionarrow_forwardShow how you might carry out the following transformation or reactions: toluene to m-chlorobenzoic acidarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardCan you please explain how to solve this problem step by step? You might consider color coding it or presenting it in a way that makes it easier for me to understand.arrow_forwardNucleophilic addition reaction of RMgX to a carbonyl compound to synthesize alcohol.arrow_forward
- Can you explain this problem to me step by step? I'm really confused. Please color-code it as well, and help me out.arrow_forwardDraw structures corresponding to each of the following names or Provide correct IUPAC names for each of the structures below. [3 ONLY] a. 1-isopropoxycyclopentene b. Diethyl ether C. 3-methyl-1-butanethiol d. OCH3 Clarrow_forward4. Choose the best reagent for carrying out the following reactions from the list below. Place the letter of the reagent(s) in the box over the reaction arrow. Use only one letter per box. OH 0 OH CH3 CH3 0 CH3 CH3 OH 賽 OCH3 H A. NaH, then CHI B. NaOCH 3, CH3OH C. m-CIC6H4CO3H D. E. warm H2SO4/H₂O F. G. H₂/Pd H. CH3MgBr in ether, then H3O+ Hg(O2CCF3)2, CH3OH PCC, CH2Cl2 I, Cl₂, H₂O J. LiAlH4 in ether, then H3O+ CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY