
Concept explainers
(a)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the given transformation is faster in polar, aprotic solvent in comparison to polar, protic solvent should be determined.
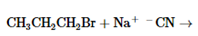
Concept Introduction:
The
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The solvents which are capable of forming hydrogen bonds due to the presence of at least one hydrogen linked with electronegative atom is known as polar protic solvents whereas the solvents in which no hydrogen atoms are linked with electronegative atom and also incapable of hydrogen bonding is known as
(b)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the given transformation is faster in polar, aprotic solvent in comparison to polar, protic solvent should be determined.
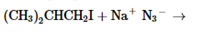
Concept Introduction:
The chemical reaction in which one functional group is substituted by another functional group is known as substitution reaction.
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The solvents which are capable of forming hydrogen bonds due to the presence of at least one hydrogen linked with electronegative atom is known as polar protic solvents whereas the solvents in which no hydrogen atoms are linked with electronegative atom and also incapable of hydrogen bonding is known as polar aprotic solvents
(c)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the given transformation is faster in polar, aprotic solvent in comparison to polar, protic solvent should be determined.
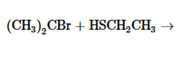
Concept Introduction:
The chemical reaction in which one functional group is substituted by another functional group is known as substitution reaction.
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The solvents which are capable of forming hydrogen bonds due to the presence of at least one hydrogen linked with electronegative atom is known as polar protic solvents whereas the solvents in which no hydrogen atoms are linked with electronegative atom and also incapable of hydrogen bonding is known as polar aprotic solvents
(d)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the given transformation is faster in polar, aprotic solvent in comparison to polar, protic solvent should be determined.

Concept Introduction:
The chemical reaction in which one functional group is substituted by another functional group is known as substitution reaction.
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The solvents which are capable of forming hydrogen bonds due to the presence of at least one hydrogen linked with electronegative atom is known as polar protic solvents whereas the solvents in which no hydrogen atoms are linked with electronegative atom and also incapable of hydrogen bonding is known as polar aprotic solvents
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
ORGANIC CHEMISTRY (LL)-PACKAGE
- When anisole is treated with excess bromine, the reaction gives a product which shows two singlets in 1H NMR. Draw the product.arrow_forward(ii) Draw a reasonable mechanism for the following reaction: CI NaOH heat OH (hint: SNAr Reaction) :arrow_forwardDraw the major product in each of the following reaction:arrow_forward
- Draw the mechanism for the following Friedel-Craft reaction. AlBr3 Brarrow_forward(a) Draw the structures of A and B in the following reaction. (i) NaNH2, NH3(1) A + B (ii) H3O+arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Consider the following decomposition reaction of N2O5(g): For the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 → NO2 + NO3 (K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Indicate whether the following rate expression is acceptable: d[N2O5] = -k₁[N₂O₂] + K¸₁[NO₂][NO3] - K¸[NO₂]³ dtarrow_forwardIn a reaction of A + B to give C, another compound other than A, B or C may appear in the kinetic equation.arrow_forwardFor the reaction 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 →> NO₂+ NO3_(K1) NO2 + NO3 → N2O5 (k-1) NO2 + NO3 → → NO2 + O2 + NO (K2) NO + N2O5- NO2 + NO2 + NO2 (K3) d[N₂O5] __2k‚k₂[N2O5] Indicate whether the following rate expression is acceptable: dt k₁₁+ k₂arrow_forward
- Given the reaction R + Q → P, indicate the rate law with respect to R, with respect to P and with respect to P.arrow_forwardSteps and explanations. Also provide, if possible, ways to adress this kind of problems in general.arrow_forwardk₁ Given the reaction A B, indicate k-1 d[A] (A). the rate law with respect to A: (B). the rate law with respect to B: d[B] dt dtarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

