
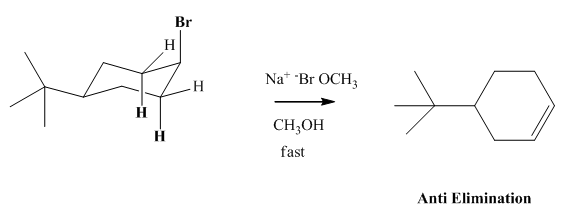
(a)
Interpretation: The reason behind rate of bimolecular elimination in
Concept introduction: Experimental evidence for
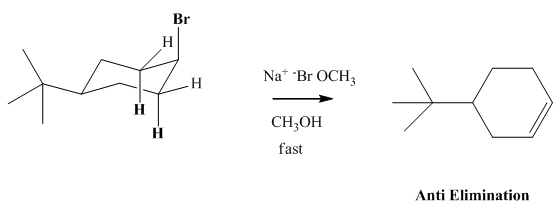
(b)
Interpretation: The formation of only specific elimination product should be explained.
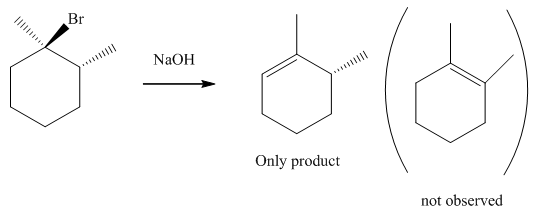
Concept introduction: Experimental evidence for
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
ORGANIC CHEMISTRY (LL)-PACKAGE
- Consider the following reactants: Br Would elimination take place at a significant rate between these reactants? Note for advanced students: by significant, we mean that the rate of elimination would be greater than the rate of competing substitution reactions. yes O no If you said elimination would take place, draw the major products in the upper drawing area. If you said elimination would take place, also draw the complete mechanism for one of the major products in the lower drawing area. If there is more than one major product, you may draw the mechanism that leads to any of them. Major Products:arrow_forwardDraw one product of an elimination reaction between the molecules below. Note: There may be several correct answers. You only need to draw one of them. You do not need to draw any of the side products of the reaction. OH + ! : ☐ + Х Click and drag to start drawing a structure.arrow_forwardFind one pertinent analytical procedure for each of following questions relating to food safety analysis. Question 1: The presence of lead, mercury and cadmium in canned tuna Question 2: Correct use of food labellingarrow_forward
- Formulate TWO key questions that are are specifically in relation to food safety. In addition to this, convert these questions into a requirement for chemical analysis.arrow_forwardWhat are the retrosynthesis and forward synthesis of these reactions?arrow_forwardWhich of the given reactions would form meso product? H₂O, H2SO4 III m CH3 CH₂ONa CH3OH || H₂O, H2SO4 CH3 1. LiAlH4, THF 2. H₂O CH3 IVarrow_forward
- What is the major product of the following reaction? O IV III HCI D = III ა IVarrow_forwardThe reaction of what nucleophile and substrate is represented by the following transition state? CH3 CH3O -Br อ δ CH3 Methanol with 2-bromopropane Methanol with 1-bromopropane Methoxide with 1-bromopropane Methoxide with 2-bromopropanearrow_forwardWhat is the stepwise mechanism for this reaction?arrow_forward
- 32. Consider a two-state system in which the low energy level is 300 J mol 1 and the higher energy level is 800 J mol 1, and the temperature is 300 K. Find the population of each level. Hint: Pay attention to your units. A. What is the partition function for this system? B. What are the populations of each level? Now instead, consider a system with energy levels of 0 J mol C. Now what is the partition function? D. And what are the populations of the two levels? E. Finally, repeat the second calculation at 500 K. and 500 J mol 1 at 300 K. F. What do you notice about the populations as you increase the temperature? At what temperature would you expect the states to have equal populations?arrow_forward30. We will derive the forms of the molecular partition functions for atoms and molecules shortly in class, but the partition function that describes the translational and rotational motion of a homonuclear diatomic molecule is given by Itrans (V,T) = = 2πmkBT h² V grot (T) 4π²IKBT h² Where h is Planck's constant and I is molecular moment of inertia. The overall partition function is qmolec Qtrans qrot. Find the energy, enthalpy, entropy, and Helmholtz free energy for the translational and rotational modes of 1 mole of oxygen molecules and 1 mole of iodine molecules at 50 K and at 300 K and with a volume of 1 m³. Here is some useful data: Moment of inertia: I2 I 7.46 x 10- 45 kg m² 2 O2 I 1.91 x 101 -46 kg m²arrow_forwardK for each reaction step. Be sure to account for all bond-breaking and bond-making steps. HI HaC Drawing Arrows! H3C OCH3 H 4 59°F Mostly sunny H CH3 HO O CH3 'C' CH3 Select to Add Arrows CH3 1 L H&C. OCH3 H H H H Select to Add Arrows Q Search Problem 30 of 20 H. H3C + :0: H CH3 CH3 20 H2C Undo Reset Done DELLarrow_forward
