
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
3rd Edition
ISBN: 9780133858501
Author: Bruice
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.14, Problem 24P
Which
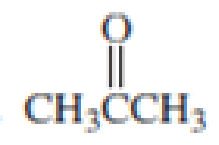
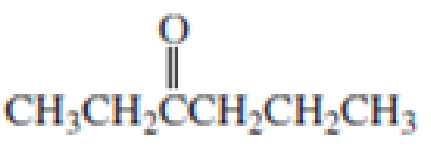
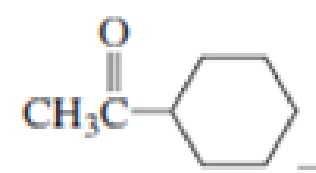
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following orbitals intersect or overlap the x-axis in the standard cartesian coordinate system used? (Select ALL correct answers.)
Group of answer choices
px
dxz
dx2-y2
py
dxy
s
Which of the following sets of elements is not a Dobereiner triad? (Choose the best answer.) Group of answer choices
Li-Na-K
Al-Ga-In
Cr-Mo-W
K-Rb-Cs
Don't used Ai solution and don't used hand raiting
Chapter 6 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
Ch. 6.1 - Draw the mechanism for the reaction of cyclohexene...Ch. 6.2 - a. How many bond orbitals are available for...Ch. 6.2 - Prob. 3PCh. 6.2 - Prob. 4PCh. 6.3 - Prob. 5PCh. 6.3 - Prob. 6PCh. 6.3 - Prob. 7PCh. 6.5 - Prob. 9PCh. 6.5 - Prob. 10PCh. 6.5 - a. What is the major product of each of the...
Ch. 6.5 - Prob. 12PCh. 6.6 - What stereoisomers are obtained from each of the...Ch. 6.6 - Prob. 14PCh. 6.8 - Prob. 15PCh. 6.10 - Name the following:Ch. 6.10 - Draw the structure for each of the following: a....Ch. 6.10 - Draw the structures for and name the seven alkynes...Ch. 6.10 - Name the following:Ch. 6.10 - Name the following:Ch. 6.11 - What hybrid orbitals are used to form the...Ch. 6.13 - Prob. 22PCh. 6.14 - Prob. 23PCh. 6.14 - Which alkyne would be the best one to use for the...Ch. 6.14 - Prob. 25PCh. 6.14 - Prob. 26PCh. 6.15 - Describe the alkyne you would start with and the...Ch. 6.15 - What are products of the following reactions?Ch. 6 - Prob. 29PCh. 6 - Prob. 30PCh. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - What is each compounds systematic name?Ch. 6 - Prob. 34PCh. 6 - Prob. 35PCh. 6 - What reagents could be used to carry out the...Ch. 6 - Prob. 37PCh. 6 - Prob. 38PCh. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Prob. 41PCh. 6 - Prob. 42PCh. 6 - Answer Problem 42 using 2-butyne as the starting...Ch. 6 - What is each compounds systematic name?Ch. 6 - Prob. 45PCh. 6 - Prob. 46PCh. 6 - Prob. 47PCh. 6 - Prob. 48PCh. 6 - Prob. 49PCh. 6 - Prob. 50PCh. 6 - Draw the keto tautomer for each of the following:Ch. 6 - Propose a mechanism for the following reaction...Ch. 6 - Prob. 53PCh. 6 - Prob. 54PCh. 6 - Prob. 55PCh. 6 - Propose a mechanism for the following reaction:Ch. 6 - Prob. 57PCh. 6 - Prob. 58PCh. 6 - Prob. 59PCh. 6 - Prob. 60PCh. 6 - Prob. 61PCh. 6 - Prob. 62P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Don't used hand raiting and don't used Ai solutionarrow_forwardGive the structure(s) of the product(s) the reaction below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise I'm struggling to see how this reaction will go! I am wondering if it will cycle on itself but I'm not sure how I drew out a decagon but I'm a bit lostarrow_forwardGive the structure(s) of the product(s) for the reactions below, and be sure to indicate any relative stereochemistry (you can assume that each of the Diels-Alder reactions will proceed with endo selectivity). Draw out relevant enantiomer(s) if they are expected to form. If no reaction is expected to occur under the indicated conditions, then write "no reaction" or NR, and explain why you would expect nothing to occur. If more than one product is formed, please indicate which one will be the major product or if they will be formed in equal amounts. In all cases, equimolar amounts of both components/reagents are present unless indicated otherwise .arrow_forward
- Calculate the residence time of strontium (Sr2+) in the world ocean, given that the average concentration of strontium in the world’s rivers is approximately 0.87 µmol L-1 (5 pts).arrow_forwardA package contains 1.33lbs of ground round. If it contains 29% fat, how many grams of fat are in the ground? arrow_forwardHow is the resonance structure formed to make the following reaction product. Please hand draw the arrows showing how the electrons move to the correct position. Do not use an AI answer. Please draw it yourself or don't bother.arrow_forward
- Part II Calculate λ max of the following compounds using wood ward- Fiecer rules a) b) c) d) e) OH OH dissolved in dioxane Br Br dissolved in methanol. NH₂ OCH 3 OHarrow_forward6. Match each of the lettered items in the column on the left with the most appropriate numbered item(s) in the column on the right. Some of the numbered items may be used more than once and some not at all. a. Z = 37 1. b. Mn 2. C. Pr element in period 5 and group 14 element in period 5 and group 15 d. S e. [Rn] 7s¹ f. d block metal 3. highest metallic character of all the elements 4. paramagnetic with 5 unpaired electrons 5. 4f36s2 6. isoelectronic with Ca²+ cation 7. an alkaline metal 8. an f-block elementarrow_forwardDraw all formal charges on the structures below as is and draw 1 resonance structure that is more stable.arrow_forward
- Part II. xiao isolated a compound TAD (Ca H 10 N₂) from tobacco and obtained its IR spectrum. Xiao proposed a chemical structure shown below: % Transmittance 4000 3500 3000 2500 2000 Wavenumber (cm-1) 1500 1000 (a) Explain why her proposed structure is inconsistent with the IR spectrum obtained (b) TAD exists as a tautomer of the structure xiao proposed. Draw the structure and explain why it is more compatible with the obtained spectrum. (C) what is the possible source for the fairly intense signal at 1621cm1arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY