
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
3rd Edition
ISBN: 9780133858501
Author: Bruice
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6.5, Problem 11P
- a. What is the major product of each of the following reactions?
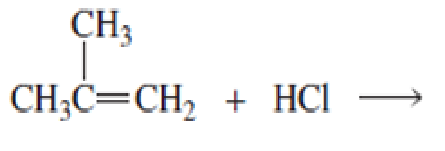
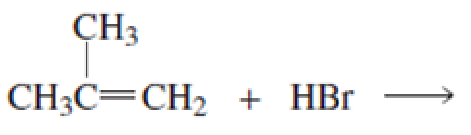
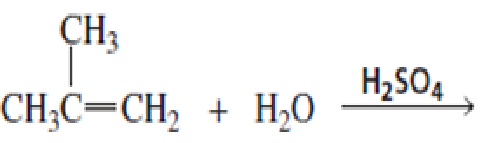
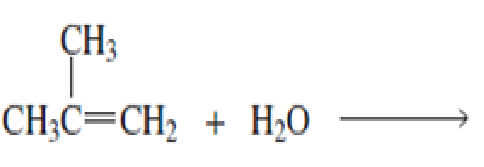
b. What do the first three reactions have in common?
c. How do the first three reactions differ?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a) What is the length of x? b) Findθ c) Find ϕ
Briefly describe the structure and bonding of graphite. Indicate some type of inorganic compound with a complex structure that forms graphite.
For c4h5n2 draw the lewis dot structure
Chapter 6 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
Ch. 6.1 - Draw the mechanism for the reaction of cyclohexene...Ch. 6.2 - a. How many bond orbitals are available for...Ch. 6.2 - Prob. 3PCh. 6.2 - Prob. 4PCh. 6.3 - Prob. 5PCh. 6.3 - Prob. 6PCh. 6.3 - Prob. 7PCh. 6.5 - Prob. 9PCh. 6.5 - Prob. 10PCh. 6.5 - a. What is the major product of each of the...
Ch. 6.5 - Prob. 12PCh. 6.6 - What stereoisomers are obtained from each of the...Ch. 6.6 - Prob. 14PCh. 6.8 - Prob. 15PCh. 6.10 - Name the following:Ch. 6.10 - Draw the structure for each of the following: a....Ch. 6.10 - Draw the structures for and name the seven alkynes...Ch. 6.10 - Name the following:Ch. 6.10 - Name the following:Ch. 6.11 - What hybrid orbitals are used to form the...Ch. 6.13 - Prob. 22PCh. 6.14 - Prob. 23PCh. 6.14 - Which alkyne would be the best one to use for the...Ch. 6.14 - Prob. 25PCh. 6.14 - Prob. 26PCh. 6.15 - Describe the alkyne you would start with and the...Ch. 6.15 - What are products of the following reactions?Ch. 6 - Prob. 29PCh. 6 - Prob. 30PCh. 6 - Prob. 31PCh. 6 - Prob. 32PCh. 6 - What is each compounds systematic name?Ch. 6 - Prob. 34PCh. 6 - Prob. 35PCh. 6 - What reagents could be used to carry out the...Ch. 6 - Prob. 37PCh. 6 - Prob. 38PCh. 6 - Prob. 39PCh. 6 - Prob. 40PCh. 6 - Prob. 41PCh. 6 - Prob. 42PCh. 6 - Answer Problem 42 using 2-butyne as the starting...Ch. 6 - What is each compounds systematic name?Ch. 6 - Prob. 45PCh. 6 - Prob. 46PCh. 6 - Prob. 47PCh. 6 - Prob. 48PCh. 6 - Prob. 49PCh. 6 - Prob. 50PCh. 6 - Draw the keto tautomer for each of the following:Ch. 6 - Propose a mechanism for the following reaction...Ch. 6 - Prob. 53PCh. 6 - Prob. 54PCh. 6 - Prob. 55PCh. 6 - Propose a mechanism for the following reaction:Ch. 6 - Prob. 57PCh. 6 - Prob. 58PCh. 6 - Prob. 59PCh. 6 - Prob. 60PCh. 6 - Prob. 61PCh. 6 - Prob. 62P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the coordination forms of Si in silicates.arrow_forwardBriefly indicate the structure and bonding of silicates.arrow_forward4 Part C Give the IUPAC name and a common name for the following ether: Spell out the full names of the compound in the indicated order separated by a comma.arrow_forward
- Try: Draw possible resonance contributing structures for the following organic species: CH3CH2NO2 [CH2CHCH2] [CH2CHCHO] [CH2CHCH2] [CH2CHNH2]arrow_forwardComplete the following synthesis. (d). H+ ง сarrow_forwardCan the target compound be efficiently synthesized in good yield from the substituted benzene of the starting material? If yes, draw the synthesis. Include all steps and all reactants.arrow_forward
- This is a synthesis question. Why is this method wrong or worse than the "correct" method? You could do it thiss way, couldn't you?arrow_forwardTry: Draw the best Lewis structure showing all non-bonding electrons and all formal charges if any: (CH3)3CCNO NCO- HN3 [CH3OH2]*arrow_forwardWhat are the major products of the following reaction? Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY