
Concept explainers
(a)
Interpretation:
The keto-enol tautomer from the given pairs should be determined.
Concept Introduction:
Enol formation:
Under acidic conditions, alkyne reacts with water to produce an enol which then immediately converts into
The formed enol and ketone are called keto-enol tautomer.
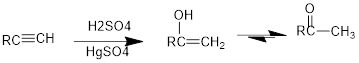
Tautomerization: It is the process of inter conversion of an enol compound to a ketone compound and therefore the process can also be called as keto-enol tautomerization.
(b)
Interpretation:
The keto-enol tautomers from the given pairs should be determined.
Concept Introduction:
Enol formation:
Under acidic conditions, alkyne reacts with water to produce an enol which then immediately converts into ketone. For terminal alkynes, there is a need of catalyst which is mercury.
The formed enol and ketone are called keto-enol tautomers.
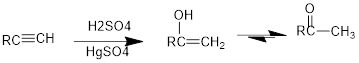
Tautomerization: It is the process of inter conversion of an enol compound to a ketone compound and therefore the process can also be called as keto-enol tautomerization.
(c)
Interpretation:
The keto-enol tautomers from the given pairs should be determined.
Concept Introduction:
Enol formation:
Under acidic conditions, alkyne reacts with water to produce an enol which then immediately converts into ketone. For terminal alkynes, there is a need of catalyst which is mercury.
The formed enol and ketone are called keto-enol tautomers.
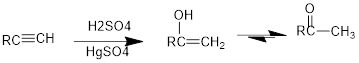
Tautomerization: It is the process of inter conversion of an enol compound to a ketone compound and therefore the process can also be called as keto-enol tautomerization.
(d)
Interpretation:
The keto-enol tautomers from the given pairs should be determined.
Concept Introduction:
Enol formation:
Under acidic conditions, alkyne reacts with water to produce an enol which then immediately converts into ketone. For terminal alkynes, there is a need of catalyst which is mercury.
The formed enol and ketone are called keto-enol tautomers.
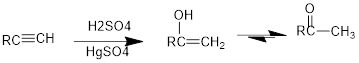
Tautomerization: It is the process of inter conversion of an enol compound to a ketone compound and therefore the process can also be called as keto-enol tautomerization.
(e)
Interpretation:
The keto-enol tautomers from the given pairs should be determined.
Concept Introduction:
Enol formation:
Under acidic conditions, alkyne reacts with water to produce an enol which then immediately converts into ketone. For terminal alkynes, there is a need of catalyst which is mercury.
The formed enol and ketone are called keto-enol tautomers.
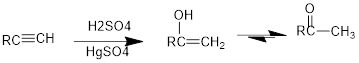
Tautomerization: It is the process of inter conversion of an enol compound to a ketone compound and therefore the process can also be called as keto-enol tautomerization.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
- For the reaction: CO2(g) + H2(g) --> CO (g) + H2O (g) Kc= 0.64 at 900 degrees celcius. if initially you start with 1.00 atmoshpere of carbon dioxide and 1 atmoshpere of hydrogen gas, what are the equilibrium partial pressuses of all species.arrow_forwardCan I please get this answered? With the correct number of significant digits.arrow_forwardDraw the Hofmann product of the dehydroiodination of this alkyl iodide. ☐ : + Explanation Check esc F1 2 3 I 88 % 5 F5 I. X © tBuOK Click and drag to sta drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Te BI BB F6 W E R Y S H Karrow_forward
- Can I please get help with this graph, if you could show exactly where it needs to pass through please.arrow_forwardDraw the condensed structure of 1,3-dihydroxy-2-pentanone. Explanation Check Click anywhere to draw the first atom of your structure. Х C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of use +arrow_forward0.500 moles of NOCl are placed into a 1.00 L vessesl at 700K and after the system comes to equilibrium, the consentration of NOCl is 0.440 M. Calculate the equilibrium constant Kc for the reaction: 2NOCL (g) --> 2NO (g) + Cl2 (g)arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
