
(a)
Interpretation:
The major product of the given reaction should be determined.
Concept Introduction:
Addition of halides to
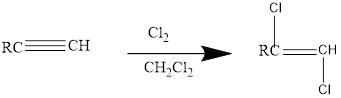
If excess halides are present, a second addition reaction also occurs.
(b)
Interpretation:
The major product of the given reaction should be determined.
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.
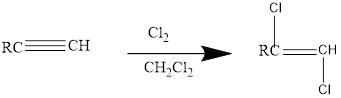
If excess halides are present, a second addition reaction also occurs.
(c)
Interpretation:
The major product of the given reaction should be determined.
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.
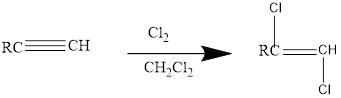
If excess halides are present, a second addition reaction also occurs.
(d)
Interpretation:
The major product of the given reaction should be determined.
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine add to the alkyne compounds. The general form of the reaction is given below.
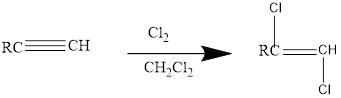
If excess halides are present, a second addition reaction also occurs.
Trending nowThis is a popular solution!

Chapter 6 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
- B. d. a hydrate 4. Give the major organic product(s) for each of the following reactions or sequences of reactions. Show all relevant stereochemistry [4 ONLY]. A. CH₂OH PCC CH2Cl2 0 H KCN HCN 2arrow_forwardPropose a synthesis of the anti-inflammatory drug Ibuprofen from benzene. Show all reagents and all intermediate structures. Assume that ortho and para isomers can be separated. (CH3)2CHCH2 CH3 CHCOOH 1buprofen be requiredarrow_forwardAssuming that no equilibria other than dissolution are involved, calculate the molar solubility of each of the following from its solubility product: (a) KHC4H4O6arrow_forward
- Answer the following by equation 1. reactio of CH3MgBr with Acetone [CH3COCH3] 2. acetal formation reaction of acetaldehyde [CH3CHO] 3. preparation of ethylmethylether [C2H5OCH3] 4. the acidity of the carboxylic acid depends and affected by the substitutions on the rest of the acid molecule: draw 2 structures of acids to show the different effects on acidity by different subsarrow_forwardConsider the reaction sequence below to answer the following questions: 0 0 0 0 0 1. NaOEt, EtOH H3O* OEt OET 2 PhCH Br heat Ph + EtOH + CO₂ CHh B C A A. The starting material A in this reaction sequence is called a a. ẞ-keto ester b. a-carboethoxy ketone C. malonic ester d. acetoacetic ester B. Conversion of A into B is a type of reaction termed a. an acylation b. an enolation C. d. an alkylation a phenylation f reactionsarrow_forward1. Refer to the compounds below to answer the following questions: CO₂Et 0 C. H O O₂N-CH2-C-CH3 0 OEt || 111 A. Indicate all the acidic hydrogens in Compounds I through IV. IV B. Indicate which hydrogens in Compound II are the most acidic. Explain your answer C. Choose the most acidic compound from Compounds I - IV. Explain your choice.arrow_forward
- Show how you would accomplish the following transformations. More than one step may be required. ow all reagents and all intermediate structures [one ONLY] A. H Br H CH3 NHz CH3 CH3 B. CH3CH2C-Br CH3CH2C-CN CH3 CH3.arrow_forwardShow how you would accomplish the following transformations. More than one step may be required. now all reagents and all intermediate structures [one ONLY] A. H Br H CH3 NHz CH3 CH3 B. CH3CH2C-Br CH3 CH3CH2C-CN CH3arrow_forwardCan I please get help with this?arrow_forward
- C. I, II, III Consider the reaction sequence below to answer the following questions: 0 0 1. NaOEt, EtOH ΕΙΟ OEt 2 Compound X CO₂Et NaOEt, EtOH CO₂Et Br Compound Y A Compound Z A. Compound X, diethyl propanedioate, is more commonly known as a. ethyl acetoacetate acetoacetic ester b. C. oxalic ester d. malonic ester B. Write the complete stepwise mechanism for the conversion of Compound X into Compound Y. Show all electron flow with arrows and draw all intermediate structures.arrow_forwardDiethyl malonate can be prepared by the following reaction sequence. Draw the structures of each of the missing intermediates in the boxes provided EtO 0 H3C 11 C 1. Br₂ PBr OH 2 H₂O 010 0 CH3CH₂OH C CH2 OEt Ha CH3CH2OH на NaCN H₂SO4 NC H₂O, heat CH2 OCH2CH3arrow_forwardShow how you would accomplish each of the following transformations. More than one step may be quired. Show all reagents and all intermediate structures. [three only] A. 0 CH3 B. C. D. H 0 0 OCH 3 CH₂CO₂CH2CH3 H3C ➤ HN C NO₂ Clarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
