
Interpretation:
The
Concept Introduction:
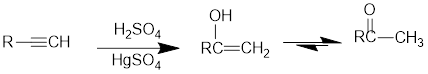
Acid Catalysed addition of water: When water is added to alkyne in the presence of an acid, the product formed will be an enol. Enol contains a double bond and a
If a carbonyl group is bonded to two alkyl groups, it is called as a ketone. The enol formed in the acid catalysed addition of water will be easily converted into a ketone.
Conversion of terminal
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EP ESSENTIAL ORG.CHEM.-MOD.MASTERING
- (2 Pts) Draw correct Lewis structures for two different molecules that have C3H6 as theirchemical formulaarrow_forwardSynthesize the following:arrow_forwardDid you report your data to the correct number of significant figures? Temperature of cold water (°C) 4.0 Temperature of hot water ("C) 87.0 Volume of cold water (mL) 94.0 Volume of hot water (mL) 78.0 Final temperature after mixing ("C) 41.0 Mass of cold water (g) 94.0 Mass of hot water (g) 78.0 Calorimeter constant (J/°C) 12.44 How to calculate the calorimeter constantarrow_forward
- please add appropriate arrows and tell me in detail where to add which or draw itarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardcan you please answer both these questions and draw the neccesaryarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



